Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action
Abstract
:1. Introduction
- Anxiety, stress and depression;
- Neurotoxicity;
- Alzheimer’s disease;
- Parkinson’s disease.
2. Materials and Methods
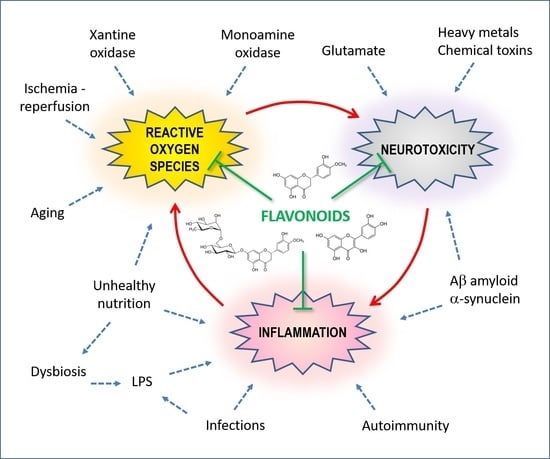
3. Oxidative Stress and Neurotoxicity
3.1. Vicious Cycles
3.2. Cell Death
4. Defense and Detoxification Systems
4.1. Flavonoids
4.2. Hesperidin and Hesperetin
4.3. Quercetin
5. Anxiety, Stress and Depression
5.1. Traditional Medicine
5.2. Post-Traumatic Stress Models
5.3. Psycho-Social Stress
5.4. Depression and Diabetes
5.5. Depression and Neuroinflammation
5.6. The Importance of Microbiota
6. Neurotoxicity
7. Alzheimer’s Disease
7.1. AD Transgenic Animal Models
7.2. Direct Pathological Effects of Aβ
7.3. Aluminum
7.4. Other AD Models
7.5. In Vitro and Bioinformatics Studies
8. Parkinson’s Disease
8.1. Hydroxydopamine-Induced PD
8.2. Rotenone-Induced PD
8.3. Ferroptosis and Neurodegeneration in PD
8.4. Transgenic Mouse
8.5. In Vitro Studies
9. Effects on Aging Models
10. Synthesis and Conclusions
10.1. Inhibition of Oxidative Stress
10.2. Protection of Cellular Structure and Function
10.3. Inflammation Modulation
Funding
Conflicts of Interest
References
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Aoi, W.; Iwasa, M.; Marunaka, Y. Metabolic functions of flavonoids: From human epidemiology to molecular mechanism. Neuropeptides 2021, 88, 102163. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, S.; Russo, G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018, 153, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Zanini, S.; Marzotto, M.; Giovinazzo, F.; Bassi, C.; Bellavite, P. Effects of dietary components on cancer of the digestive system. Crit. Rev. Food Sci. Nutr. 2015, 55, 1870–1885. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.J.; Kim, S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J. Med. Food 2018, 21, 421–432. [Google Scholar] [CrossRef]
- Babaei, F.; Mirzababaei, M.; Nassiri-Asl, M. Quercetin in Food: Possible Mechanisms of Its Effect on Memory. J. Food Sci. 2018, 83, 2280–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein, F.M.; EcheverrÃ-a, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Ahmad, J.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar] [CrossRef]
- Meng-Zhen, S.; Ju, L.; Lan-Chun, Z.; Cai-Feng, D.; Shu-da, Y.; Hao-Fei, Y.; Wei-Yan, H. Potential therapeutic use of plant flavonoids in AD and PD. Heliyon 2022, 8, e11440. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Gao, Y.; Nie, K.; Wang, H.; Su, H.; Wang, Z.; Lu, F.; Huang, W.; Dong, H. Antidepressant Potential of Quercetin and its Glycoside Derivatives: A Comprehensive Review and Update. Front. Pharmacol. 2022, 13, 865376. [Google Scholar] [CrossRef]
- Minocha, T.; Birla, H.; Obaid, A.A.; Rai, V.; Sushma, P.; Shivamallu, C.; Moustafa, M.; Al-Shehri, M.; Al-Emam, A.; Tikhonova, M.A.; et al. Flavonoids as Promising Neuroprotectants and Their Therapeutic Potential against Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 6038996. [Google Scholar] [CrossRef]
- Guo, C.; Wang, W.J.; Liao, Y.C.; Zhao, C.; Yin, Y.; Yao, M.N.; Ding, Y.; Wang, J.W. Effect and Mechanisms of Quercetin for Experimental Focal Cerebral Ischemia: A Systematic Review and Meta-Analysis. Oxid. Med. Cell. Longev. 2022, 2022, 9749461. [Google Scholar] [CrossRef]
- Cho, J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch. Pharm. Res. 2006, 29, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M. Oxidative stress in developmental brain disorders. Neuropathology 2009, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Caksen, H.; Ozkan, M.; Cemek, M.; Cemek, F. Oxidant and antioxidant status in children with subacute sclerosing panencephalitis. J. Child Neurol. 2014, 29, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Gold, R.; Linker, R.A. Mechanisms of Oxidative Damage in Multiple Sclerosis and Neurodegenerative Diseases: Therapeutic Modulation via Fumaric Acid Esters. Int. J. Mol. Sci. 2012, 13, 11783–11803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Michalska, P.; León, R. When It Comes to an End: Oxidative Stress Crosstalk with Protein Aggregation and Neuroinflammation Induce Neurodegeneration. Antioxidants 2020, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A. Molecular Pathophysiological Mechanisms in Huntington’s Disease. Biomedicines 2022, 10, 1432. [Google Scholar] [CrossRef]
- Irfan, Z.; Khanam, S.; Karmakar, V.; Firdous, S.M.; El Khier, B.; Khan, I.; Rehman, M.U.; Khan, A. Pathogenesis of Huntington’s Disease: An Emphasis on Molecular Pathways and Prevention by Natural Remedies. Brain Sci. 2022, 12, 1389. [Google Scholar] [CrossRef]
- Jaiswal, P.; Mandal, M.; Mishra, A. Effect of hesperidin on fluoride-induced neurobehavioral and biochemical changes in rats. J. Biochem. Mol. Toxicol 2020, 34, e22575. [Google Scholar] [CrossRef]
- Leonard, B.E. The HPA and immune axes in stress: The involvement of the serotonergic system. Eur. Psychiatry 2005, 20 (Suppl. 3), S302–S306. [Google Scholar] [CrossRef]
- Bellavite, P. The superoxide-forming enzymatic system of phagocytes. Free Radic. Biol. Med. 1988, 4, 225–261. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L.; Levi, G. Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog. Neurobiol. 1998, 54, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Tse, J.K.Y. Gut Microbiota, Nitric Oxide, and Microglia as Prerequisites for Neurodegenerative Disorders. ACS Chem. Neurosci. 2017, 8, 1438–1447. [Google Scholar] [CrossRef]
- Bianca, V.D.; Dusi, S.; Bianchini, E.; Dal Pra, I.; Rossi, F. beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J. Biol. Chem. 1999, 274, 15493–15499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strunecka, A.; Blaylock, R.L.; Patocka, J.; Strunecky, O. Immunoexcitotoxicity as the central mechanism of etiopathology and treatment of autism spectrum disorders: A possible role of fluoride and aluminum. Surg. Neurol. Int. 2018, 9, 74. [Google Scholar] [CrossRef]
- Jovanova-Nesic, K.; Shoenfeld, Y.; Spector, N.H. Aluminum excytotoxicity and neuroautotoimmunity: The role of the brain expression of CD32+ (FcgammaRIIa), ICAM-1+ and CD3xi in aging. Curr. Aging Sci. 2012, 5, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Levite, M. Glutamate, T cells and multiple sclerosis. J. Neural. Transm. 2017, 124, 775–798. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lin, T.Y.; Lu, C.W.; Huang, S.K.; Wang, Y.C.; Chou, S.S.; Wang, S.J. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology 2015, 50, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Oliveira, P.J.; Dias, A.; Malva, J.O. Quercetin, kaempferol and biapigenin from Hypericum perforatum are neuroprotective against excitotoxic insults. Neurotox. Res. 2008, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Najmi, A.K.; Akhtar, M. A Natural Phenolic Compound Quercetin Showed the Usefulness by Targeting Inflammatory, Oxidative Stress Markers and Augment 5-HT Levels in One of the Animal Models of Depression in Mice. Drug Res. 2019, 69, 392–400. [Google Scholar] [CrossRef]
- Cardozo, V.; Vaamonde, L.; Parodi-Talice, A.; Zuluaga, M.J.; Agrati, D.; Portela, M.; Lima, A.; Blasina, F.; Dajas, F.; Bedó, G. Multitarget neuroprotection by Quercetin: Changes in gene expression in two perinatal asphyxia models. Neurochem. Int. 2021, 147, 105064. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Adhami, V.M.; Mukhtar, H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr. Relat. Cancer 2010, 17, R39–R52. [Google Scholar] [CrossRef] [Green Version]
- Ge, C.; Zhang, S.; Mu, H.; Zheng, S.; Tan, Z.; Huang, X.; Xu, C.; Zou, J.; Zhu, Y.; Feng, D.; et al. Emerging Mechanisms and Disease Implications of Ferroptosis: Potential Applications of Natural Products. Front. Cell Dev. Biol. 2021, 9, 774957. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, P.; Mohan, T.; Ravi, D.B.; Kishore Kumar, S.N.; Srinivasan, A.; Chakrapani, L.N.; Singh, A.; Varadharaj, S.; Kalaiselvi, P. Targeting the Nrf2/ARE Signalling Pathway to Mitigate Isoproterenol-Induced Cardiac Hypertrophy: Plausible Role of Hesperetin in Redox Homeostasis. Oxid. Med. Cell. Longev. 2020, 2020, 9568278. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Nrf2/HO-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxid. Med. Cell. Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef] [Green Version]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, G.; Shao, N.; Qin, Y.; Chen, Q.; Wang, Y.; Zhou, P.; Cai, B. Thioredoxin-interacting protein (TXNIP) as a target for Alzheimer’s disease: Flavonoids and phenols. Inflammopharmacology 2021, 29, 1317–1329. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Khanbabapour Sasi, A.; Taheri, M.; Ayatollahi, S.A. The impact of the phytotherapeutic agent quercetin on expression of genes and activity of signaling pathways. Biomed. Pharmacother. 2021, 141, 111847. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Li, H.; Liu, J.; Zhang, P.; Cheng, Y.; Qin, X.; Hu, Y.; Wei, Y. The neuroprotective effects of isoquercitrin purified from apple pomace by high-speed countercurrent chromatography in the MPTP acute mouse model of Parkinson’s disease. Food Funct. 2021, 12, 6091–6101. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, T.; Qi, B.; Kong, L.; Jiao, Y.; Cao, Y.; Zhang, J.; Yang, J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3 inducing rats model of Alzheimer’s disease. J. Ethnopharmacol. 2016, 179, 162–169. [Google Scholar] [CrossRef]
- Grasso, M.; Caruso, G.; Godos, J.; Bonaccorso, A.; Carbone, C.; Castellano, S.; Currenti, W.; Grosso, G.; Musumeci, T.; Caraci, F. Improving Cognition with Nutraceuticals Targeting TGF-beta1 Signaling. Antioxidants 2021, 10, 1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxid. Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure-Activity Relationship-A Quantum Chemical Study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, L.; Sun, C.; Zhao, D.; Tang, H. Studies on the structure-activity relationship and interaction mechanism of flavonoids and xanthine oxidase through enzyme kinetics, spectroscopy methods and molecular simulations. Food Chem. 2020, 323, 126807. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of Citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [Green Version]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Guler, H.I.; Tatar, G.; Yildiz, O.; Belduz, A.O.; Kolayli, S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. Arch. Microbiol. 2021, 203, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, Y.; Yang, D.; He, Z.; Lin, X.; Wang, G.; Yu, W. Simultaneous determination of phenolic metabolites in Chinese citrus and grape cultivars. PeerJ 2020, 8, e9083. [Google Scholar] [CrossRef]
- Gao, C.; Kong, S.; Guo, B.; Liang, X.; Duan, H.; Li, D. Antidepressive Effects of Taraxacum Officinale in a Mouse Model of Depression Are Due to Inhibition of Corticosterone Levels and Modulation of Mitogen-Activated Protein Kinase Phosphatase-1 (Mkp-1) and Brain-Derived Neurotrophic Factor (Bdnf) Expression. Med. Sci. Monit. 2019, 25, 389–394. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, X.; Chen, P.; Chen, T.; Peng, W.; Su, W. Integrating Pharmacology and Gut Microbiota Analysis to Explore the Mechanism of Citri Reticulatae Pericarpium Against Reserpine-Induced Spleen Deficiency in Rats. Front. Pharmacol. 2020, 11, 586350. [Google Scholar] [CrossRef]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-kappaB Signaling in an Abeta Mouse Model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef]
- Justin-Thenmozhi, A.; Dhivya, B.M.; Kiruthika, R.; Manivasagam, T.; Borah, A.; Essa, M.M. Attenuation of Aluminum Chloride-Induced Neuroinflammation and Caspase Activation Through the AKT/GSK-3Î2 Pathway by Hesperidin in Wistar Rats. Neurotox. Res. 2018, 34, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Caro, G.; Borges, G.; van der Hooft, J.; Clifford, M.N.; Del, R.D.; Lean, M.E.; Roberts, S.A.; Kellerhals, M.B.; Crozier, A. Orange juice (poly)phenols are highly bioavailable in humans. Am. J. Clin. Nutr. 2014, 100, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Caro, G.; Clifford, M.N.; Polyviou, T.; Ludwig, I.A.; Alfheeaid, H.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Plasma pharmacokinetics of (poly)phenol metabolites and catabolites after ingestion of orange juice by endurance trained men. Free Radic. Biol. Med. 2020, 160, 784–795. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Borges, G.; Ky, I.; Ribas, A.; Calani, L.; Del, R.D.; Clifford, M.N.; Roberts, S.A.; Crozier, A. In vitro colonic catabolism of orange juice (poly)phenols. Mol. Nutr. Food Res. 2015, 59, 465–475. [Google Scholar] [CrossRef]
- Sharma, R. Polyphenols in Health and Disease: Practice and Mechanisms of Benefits. In Polyphenols in Health and Disease; Academic Press: Cambridge, MA, USA, 2014; pp. 757–778. [Google Scholar]
- Li, Y.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul. Toxicol. Pharmacol. 2019, 105, 77–85. [Google Scholar] [CrossRef]
- Haggag, Y.A.; El-Ashmawy, N.E.; Okasha, K.M. Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection? Med. Hypotheses 2020, 144, 109957. [Google Scholar] [CrossRef]
- Nagasako-Akazome, Y. Chapter 58—Safety of high and long-term intake of polyphenols. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 747–756. [Google Scholar]
- Dupuis, J.; Laurin, P.; Tardif, J.C.; Hausermann, L.; Rosa, C.; Guertin, M.C.; Thibaudeau, K.; Gagnon, L.; Cesari, F.; Robitaille, M.; et al. Fourteen-days Evolution of COVID-19 Symptoms During the Third Wave in Non-vaccinated Subjects and Effects of Hesperidin Therapy: A randomized, double-blinded, placebo-controlled study. Evid. Based Complement Altern. Med. 2022, 2022, 3125662. [Google Scholar] [CrossRef]
- Kumar, S.; Paul, P.; Yadav, P.; Kaul, R.; Maitra, S.S.; Jha, S.K.; Chaari, A. A multi-targeted approach to identify potential flavonoids against three targets in the SARS-CoV-2 life cycle. Comput. Biol. Med. 2022, 142, 105231. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [PubMed]
- Formica, J.V.; Regelson, W. Review of the biology of Quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar]
- Moon, D.G.; Cheon, J.; Yoon, D.H.; Park, H.S.; Kim, H.K.; Kim, J.J.; Koh, S.K. Allium sativum potentiates suicide gene therapy for murine transitional cell carcinoma. Nutr. Cancer 2000, 38, 98–105. [Google Scholar] [CrossRef]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Henriquez, G.; Gomez, A.; Guerrero, E.; Narayan, M. Potential Role of Natural Polyphenols against Protein Aggregation Toxicity: In Vitro, In Vivo, and Clinical Studies. ACS Chem. Neurosci. 2020, 11, 2915–2934. [Google Scholar] [CrossRef]
- Mouffouk, C.; Mouffouk, S.; Mouffouk, S.; Hambaba, L.; Haba, H. Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CL(pro) and PL(pro)), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2). Eur. J. Pharmacol. 2021, 891, 173759. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef]
- Li, Y.; Paxton, J.W. The effects of flavonoids on the ABC transporters: Consequences for the pharmacokinetics of substrate drugs. Expert. Opin. Drug Metab. Toxicol. 2013, 9, 267–285. [Google Scholar] [CrossRef]
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Huang, J.; Cheng, Y.C.; Zhang, Y.W. Traditional Chinese Medicine in Depression Treatment: From Molecules to Systems. Front. Pharmacol. 2020, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C. The Role of Dopamine in Inflammation-Associated Depression: Mechanisms and Therapeutic Implications. Curr. Top Behav. Neurosci. 2017, 31, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-Lopez-Alberca, C.; Gonzalez-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef]
- Morgese, M.G.; Trabace, L. Monoaminergic System Modulation in Depression and Alzheimer’s Disease: A New Standpoint? Front. Pharmacol. 2019, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Holsboer, F.; Ising, M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. Eur. J. Pharmacol. 2008, 583, 350–357. [Google Scholar] [CrossRef]
- Maguire, J. Neuroactive Steroids and GABAergic Involvement in the Neuroendocrine Dysfunction Associated With Major Depressive Disorder and Postpartum Depression. Front. Cell Neurosci. 2019, 13, 83. [Google Scholar] [CrossRef] [Green Version]
- Silberstein, S.; Liberman, A.C.; Dos Santos Claro, P.A.; Ugo, M.B.; Deussing, J.M.; Arzt, E. Stress-Related Brain Neuroinflammation Impact in Depression: Role of the Corticotropin-Releasing Hormone System and P2X7 Receptor. Neuroimmunomodulation 2021, 28, 52–60. [Google Scholar] [CrossRef]
- Qiu, W.; Cai, X.; Zheng, C.; Qiu, S.; Ke, H.; Huang, Y. Update on the Relationship Between Depression and Neuroendocrine Metabolism. Front. Neurosci. 2021, 15, 728810. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, L.; Wang, D.; Guan, S.; Lu, P.; Xu, X.; Xu, H. Influence of early life stress on depression: From the perspective of neuroendocrine to the participation of gut microbiota. Aging 2021, 13, 25588–25601. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Mudra Rakshasa-Loots, A.; Whalley, H.C.; Vera, J.H.; Cox, S.R. Neuroinflammation in HIV-associated depression: Evidence and future perspectives. Mol. Psychiatry 2022, 27, 3619–3632. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, K.L.O.; Salla, D.H.; de Oliveira, M.P.; da Silva, L.E.; Dela Vedova, L.M.; Mendes, T.F.; Bressan, C.B.C.; Costa, A.B.; da Silva, M.R.; Reus, G.Z.; et al. The impact of obesity-related neuroinflammation on postpartum depression: A narrative review. Int. J. Dev. Neurosci. 2022, 82, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.; MacPherson, A.; Bambico, F. Neuroinflammation and neuroprogression in depression: Effects of alternative drug treatments. Brain Behav. Immun. Health 2022, 26, 100554. [Google Scholar] [CrossRef] [PubMed]
- Leprun, P.M.B.; Clarke, G. The gut microbiome and pharmacology: A prescription for therapeutic targeting of the gut-brain axis. Curr. Opin. Pharmacol. 2019, 49, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Donoso, F.; Egerton, S.; Bastiaanssen, T.F.S.; Fitzgerald, P.; Gite, S.; Fouhy, F.; Ross, R.P.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Polyphenols selectively reverse early-life stress-induced behavioural, neurochemical and microbiota changes in the rat. Psychoneuroendocrinology 2020, 116, 104673. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Gan, H.; Wang, L.; Huang, J.; Chen, J. Polyphenols in edible herbal medicine: Targeting gut-brain interactions in depression-associated neuroinflammation. Crit. Rev Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Signorini, A. The Emerging Science of Homeopathy: Complexity, Biodynamics, and Nanopharmacology; North Atlantic: Berkeley, CA, USA, 2002. [Google Scholar]
- Bellavite, P. La complessità in immunologia, dalla molecola al gregge (Complexity in immunology, from molecule to herd). PNEI Review 2018, 1/2018, 18–40. [Google Scholar] [CrossRef]
- Bellavite, P. La Complessità in Medicina. Fondamenti di un Approccio Sistemico e Dinamico alla Salute, alla Malattia e Alle Terapie Integrate; Tecniche Nuove: Milano, Italy, 2009. [Google Scholar]
- Neto, F.L.; Borges, G.; Torres-Sanchez, S.; Mico, J.A.; Berrocoso, E. Neurotrophins role in depression neurobiology: A review of basic and clinical evidence. Curr. Neuropharmacol. 2011, 9, 530–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.Y.; Chen, K.M.; Belcastro, F. Dietary patterns and depression risk in older adults: Systematic review and meta-analysis. Nutr. Rev. 2021, 79, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Rahe, C.; Unrath, M.; Berger, K. Dietary patterns and the risk of depression in adults: A systematic review of observational studies. Eur. J. Nutr. 2014, 53, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Corbi, G.; Maes, M.; Scapagnini, G.; Davinelli, S. Exploring the Impact of Flavonoids on Symptoms of Depression: A Systematic Review and Meta-Analysis. Antioxidants 2021, 10, 1644. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.A.; Gonzalez-Sarrias, A.; Laparra-Llopis, J.M.; Schneider, C.; Espin, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7937. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Goutman, J.D.; Waxemberg, M.D.; Donate-Oliver, F.; Pomata, P.E.; Calvo, D.J. Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur. J. Pharmacol. 2003, 461, 79–87. [Google Scholar] [CrossRef]
- Goutman, J.D.; Calvo, D.J. Studies on the mechanisms of action of picrotoxin, quercetin and pregnanolone at the GABA rho 1 receptor. Br. J. Pharmacol. 2004, 141, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, R.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Islam, M.S.; Dey, D.; Jain, D.; Faria, F.; Akbor, R.; Atolani, O.; et al. Quercetin and/or Ascorbic Acid Modulatory Effect on Phenobarbital-Induced Sleeping Mice Possibly through GABA(A) and GABA(B) Receptor Interaction Pathway. Pharmaceuticals 2021, 14, 721. [Google Scholar] [CrossRef]
- Islam, M.S.; Hossain, R.; Ahmed, T.; Rahaman, M.M.; Al-Khafaji, K.; Khan, R.A.; Sarkar, C.; Bappi, M.H.; de Andrade, E.M.; Araujo, I.M.; et al. Anxiolytic-like Effect of Quercetin Possibly through GABA Receptor Interaction Pathway: In Vivo and In Silico Studies. Molecules 2022, 27, 149. [Google Scholar] [CrossRef]
- Marder, M.; Viola, H.; Wasowski, C.; Fernandez, S.; Medina, J.H.; Paladini, A.C. 6-methylapigenin and hesperidin: New valeriana flavonoids with activity on the CNS. Pharmacol. Biochem. Behav. 2003, 75, 537–545. [Google Scholar] [CrossRef]
- Fernandez, S.; Wasowski, C.; Paladini, A.C.; Marder, M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004, 77, 399–404. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Wasowski, C.; Paladini, A.C.; Marder, M. Synergistic interaction between hesperidin, a natural flavonoid, and diazepam. Eur. J. Pharmacol. 2005, 512, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, L.M.; Wasowski, C.; Paladini, A.C.; Marder, M. Opioid receptors are involved in the sedative and antinociceptive effects of hesperidin as well as in its potentiation with benzodiazepines. Eur. J. Pharmacol. 2008, 580, 306–313. [Google Scholar] [CrossRef]
- Guzman-Gutierrez, S.L.; Navarrete, A. Pharmacological exploration of the sedative mechanism of hesperidin identified as the active principle of Citrus sinensis flowers. Planta Med. 2009, 75, 295–301. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; Zamilpa, A.; Gonzalez-Cortazar, M.; Reyes-Chilpa, R.; Leon, E.; Garcia, M.P.; Tortoriello, J.; Huerta-Reyes, M. Antidepressant effect and pharmacological evaluation of standardized extract of flavonoids from Byrsonima crassifolia. Phytomedicine 2011, 18, 1255–1261. [Google Scholar] [CrossRef]
- Ashafaq, M.; Varshney, L.; Khan, M.H.; Salman, M.; Naseem, M.; Wajid, S.; Parvez, S. Neuromodulatory effects of hesperidin in mitigating oxidative stress in streptozotocin induced diabetes. Biomed. Res. Int. 2014, 2014, 249031. [Google Scholar] [CrossRef] [Green Version]
- Santos, G.; Giraldez-Alvarez, L.D.; Avila-Rodriguez, M.; Capani, F.; Galembeck, E.; Neto, A.G.; Barreto, G.E.; Andrade, B. SUR1 Receptor Interaction with Hesperidin and Linarin Predicts Possible Mechanisms of Action of Valeriana officinalis in Parkinson. Front. Aging Neurosci. 2016, 8, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, L.C.; de Gomes, M.G.; Goes, A.T.; Del Fabbro, L.; Filho, C.B.; Boeira, S.P.; Jesse, C.R. Evidence for the involvement of the serotonergic 5-HT(1A) receptors in the antidepressant-like effect caused by hesperidin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; de Gomes, M.G.; Goes, A.T.; Filho, C.B.; Del Fabbro, L.; Antunes, M.S.; Souza, L.C.; Boeira, S.P.; Jesse, C.R. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res. Bull. 2014, 104, 19–26. [Google Scholar] [CrossRef]
- Lee, B.; Choi, G.M.; Sur, B. Antidepressant-Like Effects of Hesperidin in Animal Model of Post-Traumatic Stress Disorder. Chin. J. Integr. Med. 2021, 27, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wasowski, C.; Loscalzo, L.M.; Higgs, J.; Marder, M. Chronic intraperitoneal and oral treatments with hesperidin induce central nervous system effects in mice. Phytother. Res. 2012, 26, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shao, H.; Zhang, X.; Qin, B. Hesperidin Alleviates Lipopolysaccharide-Induced Neuroinflammation in Mice by Promoting the miRNA-132 Pathway. Inflammation 2016, 39, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-kappaB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Abbasi, M.M.; Salari, A.A. Hesperidin attenuates depression-related symptoms in mice with mild traumatic brain injury. Life Sci. 2018, 213, 198–205. [Google Scholar] [CrossRef]
- Nandeesh, R.; Vijayakumar, S.; Munnolli, A.; Alreddy, A.; Veerapur, V.P.; Chandramohan, V.; Manjunatha, E. Bioactive phenolic fraction of Citrus maxima abate lipopolysaccharide-induced sickness behaviour and anorexia in mice: In-silico molecular docking and dynamic studies of biomarkers against NF-κB. Biomed. Pharmacother. 2018, 108, 1535–1545. [Google Scholar] [CrossRef]
- Fu, H.; Liu, L.; Tong, Y.; Li, Y.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Xiao, D.; Wen, K.; et al. The antidepressant effects of hesperidin on chronic unpredictable mild stress-induced mice. Eur. J. Pharmacol. 2019, 853, 236–246. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, M.E.; Cho, J.H.; Lee, Y.; Lee, J.; Park, Y.D.; Lee, J.S. Hesperetin inhibits neuroinflammation on microglia by suppressing inflammatory cytokines and MAPK pathways. Arch. Pharm. Res. 2019, 42, 695–703. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Z.; Liu, H.; Jia, B.; Wang, Y.; Cao, M.; Song, R.; Zhang, Z.; Bian, Y. The Anti-Depressive Effects of Hesperidin and the Relative Mechanisms Based on the NLRP3 Inflammatory Signaling Pathway. Front. Pharmacol. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Kwatra, M.; Ahmed, S.; Gawali, B.; Panda, S.R.; Naidu, V.G.M. Hesperidin alleviates chronic restraint stress and lipopolysaccharide-induced Hippocampus and Frontal cortex damage in mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE signaling. Neurochem. Int. 2020, 140, 104835. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.D.; Kim, J.; Choi, Y.; Ekanayake, P.; Ahn, M.; Shin, T. Hesperidin improves motor disability in rat spinal cord injury through anti-inflammatory and antioxidant mechanism via Nrf-2/HO-1 pathway. Neurosci. Lett. 2020, 715, 134619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, H.; Liu, Y.; Chen, Y.; Liu, Y.; Yin, X. The Antidepressant-Like Effects of Hesperidin in Streptozotocin-Induced Diabetic Rats by Activating Nrf2/ARE/Glyoxalase 1 Pathway. Front. Pharmacol. 2020, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.M.; Zhang, M.Y.; Chen, Y.J.; Liu, Y.W. Hesperetin ameliorates diabetes-associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab. Brain Dis. 2021, 36, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, H.; Deng, Z.; Yan, C.; Liu, Y.; Yin, X. Hesperidin Exerts Anxiolytic-like Effects in Rats with Streptozotocin-Induced Diabetes via PKA/CREB Signaling. Curr. Mol. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Anjaneyulu, M.; Chopra, K.; Kaur, I. Antidepressant activity of quercetin, a bioflavonoid, in streptozotocin-induced diabetic mice. J. Med. Food 2003, 6, 391–395. [Google Scholar] [CrossRef]
- Grundmann, O.; Kelber, O.; Butterweck, V. Effects of St. John’s wort extract and single constituents on stress-induced hyperthermia in mice. Planta Med. 2006, 72, 1366–1371. [Google Scholar] [CrossRef]
- Sakakibara, H.; Yoshino, S.; Kawai, Y.; Terao, J. Antidepressant-like effect of onion (Allium cepa L.) powder in a rat behavioral model of depression. Biosci. Biotechnol. Biochem. 2008, 72, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bureau, G.; Longpre, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Ubgade, A.; Quazi, M.; Umathe, S.; Mundhada, D. Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 955–960. [Google Scholar] [CrossRef]
- Rinwa, P.; Kumar, A. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience 2013, 255, 86–98. [Google Scholar] [CrossRef]
- Holzmann, I.; da Silva, L.M.; Correa da Silva, J.A.; Steimbach, V.M.; de Souza, M.M. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol. Biochem. Behav. 2015, 136, 55–63. [Google Scholar] [CrossRef]
- Demir, E.A.; Gergerlioglu, H.S.; Oz, M. Antidepressant-like effects of quercetin in diabetic rats are independent of hypothalamic-pituitary-adrenal axis. Acta Neuropsychiatr. 2016, 28, 23–30. [Google Scholar] [CrossRef]
- Samad, N.; Saleem, A.; Yasmin, F.; Shehzad, M.A. Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in male mice. Physiol. Res. 2018, 67, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018, 9, 1383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ning, L.; Wang, J. Dietary quercetin attenuates depressive-like behaviors by inhibiting astrocyte reactivation in response to stress. Biochem. Biophys. Res. Commun. 2020, 533, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Liu, M.; Cui, W.; Yang, L.; Zhang, C.N. Quercetin affects shoaling and anxiety behaviors in zebrafish: Involvement of neuroinflammation and neuron apoptosis. Fish Shellfish Immunol. 2020, 105, 359–368. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, J.; Wu, X.; Song, L.; Wang, Y.; Gong, M.; Li, B. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021, 1772, 147661. [Google Scholar] [CrossRef]
- Guan, T.; Cao, C.; Hou, Y.; Li, Y.; Wei, X.; Li, S.; Jia, S.; Zhao, X. Effects of quercetin on the alterations of serum elements in chronic unpredictable mild stress-induced depressed rats. Biometals 2021, 34, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Wu, Z.; Yu, X.; Yin, Y.; Qian, S.; Wang, Z.; Huang, J.; Wang, W.; Liu, T.; et al. Quercitrin Rapidly Alleviated Depression-like Behaviors in Lipopolysaccharide-Treated Mice: The Involvement of PI3K/AKT/NF-kappaB Signaling Suppression and CREB/BDNF Signaling Restoration in the Hippocampus. ACS Chem. Neurosci. 2021, 12, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Du, G.; Wu, H.; Gao, X.; Yang, Z.; Liu, B.; Cui, S. Protective effects of quercetin on traumatic brain injury induced inflammation and oxidative stress in cortex through activating Nrf2/HO-1 pathway. Restor. Neurol. Neurosci. 2021, 39, 73–84. [Google Scholar] [CrossRef]
- Chen, F.; Sun, J.; Chen, C.; Zhang, Y.; Zou, L.; Zhang, Z.; Chen, M.; Wu, H.; Tian, W.; Liu, Y.; et al. Quercetin Mitigates Methamphetamine-Induced Anxiety-Like Behavior Through Ameliorating Mitochondrial Dysfunction and Neuroinflammation. Front. Mol. Neurosci. 2022, 15, 829886. [Google Scholar] [CrossRef]
- Ugwu, P.I.; Ben-Azu, B.; Ugwu, S.U.; Uruaka, C.I.; Nworgu, C.C.; Okorie, P.O.; Okafor, K.O.; Anachuna, K.K.; Elendu, M.U.; Ugwu, A.O.; et al. Preventive putative mechanisms involved in the psychopathologies of mice passively coping with psychosocial defeat stress by quercetin. Brain Res. Bull. 2022, 183, 127–141. [Google Scholar] [CrossRef]
- Singh, S.; Sahu, K.; Kapil, L.; Singh, C.; Singh, A. Quercetin ameliorates lipopolysaccharide-induced neuroinflammation and oxidative stress in adult zebrafish. Mol. Biol. Rep. 2022, 49, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.C.; Fernandez, S.P.; Loscalzo, L.M.; Wasowski, C.; Paladini, A.C.; Marder, M.; Medina, J.H.; Viola, H. Hesperidin, a flavonoid glycoside with sedative effect, decreases brain pERK1/2 levels in mice. Pharmacol. Biochem. Behav. 2009, 92, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, H.; Zhou, C.; Jia, H.; Ma, Z.; Zou, Z. Identification of the chemical constituents in aqueous extract of Zhi-Qiao and evaluation of its antidepressant effect. Molecules 2015, 20, 6925–6940. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Borges Filho, C.; Giacomeli, R.; Alvater, E.E.; Del Fabbro, L.; Antunes Mda, S.; de Gomes, M.G.; Goes, A.T.; Souza, L.C.; Boeira, S.P.; et al. Evidence for the Involvement of Potassium Channel Inhibition in the Antidepressant-Like Effects of Hesperidin in the Tail Suspension Test in Mice. J. Med. Food 2015, 18, 818–823. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, K.; Kawai, Y.; Terao, J. Suppressive effect of quercetin on acute stress-induced hypothalamic-pituitary-adrenal axis response in Wistar rats. J. Nutr. Biochem. 2010, 21, 374–380. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Sobarzo-Sanchez, E.; Uriarte, E.; Khatkar, A. Quercetin and Related Chromenone Derivatives as Monoamine Oxidase Inhibitors: Targeting Neurological and Mental Disorders. Molecules 2019, 24, 418. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, S.; Hara, A.; Sakakibara, H.; Kawabata, K.; Tokumura, A.; Ishisaka, A.; Kawai, Y.; Terao, J. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition 2011, 27, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Reza, M.I.; Yadav, H.; Gayen, J.R. Hesperidin inhibits NOX4 mediated oxidative stress and inflammation by upregulating SIRT1 in experimental diabetic neuropathy. Exp. Gerontol. 2022, 172, 112064. [Google Scholar] [CrossRef] [PubMed]
- Taile, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.F.; Mazzio, E.A. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc. Soc. Exp Biol. Med. 1998, 218, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflammation 2019, 16, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daulatzai, M.A. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem. Res. 2014, 39, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, L.J. Adverse Reactions to Vaccination: From Anaphylaxis to Autoimmunity. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 279–290. [Google Scholar] [CrossRef]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Dehner, C.; Fine, R.; Kriegel, M.A. The microbiome in systemic autoimmune disease: Mechanistic insights from recent studies. Curr. Opin. Rheumatol. 2019, 31, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.K.; Ou, Y.C.; Raung, S.L.; Lai, C.Y.; Liao, S.L.; Chen, C.J. Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci. 2010, 86, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; McManus, R.M.; Latz, E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018, 19, 610–621. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Metz, L.M.; Yong, V.W. Focus on the gut-brain axis: Multiple sclerosis, the intestinal barrier and the microbiome. World J. Gastroenterol. 2018, 24, 4217–4223. [Google Scholar] [CrossRef]
- Brambilla, L.; Martorana, F.; Rossi, D. Astrocyte signaling and neurodegeneration: New insights into CNS disorders. Prion 2013, 7, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Agorastos, A.; Bozikas, V.P. Gut microbiome and adaptive immunity in schizophrenia. Psychiatriki 2019, 30, 189–192. [Google Scholar] [CrossRef]
- Ashwood, P.; Anthony, A.; Pellicer, A.A.; Torrente, F.; Walker-Smith, J.A.; Wakefield, A.J. Intestinal lymphocyte populations in children with regressive autism: Evidence for extensive mucosal immunopathology. J. Clin. Immunol. 2003, 23, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.J.; Puleston, J.M.; Montgomery, S.M.; Anthony, A.; O’Leary, J.J.; Murch, S.H. Review article: The concept of entero-colonic encephalopathy, autism and opioid receptor ligands. Aliment. Pharmacol. Ther. 2002, 16, 663–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, J.R.; Kang, S.Y.; Kim, S.E.; Lee, S.J.; Lee, Y.C.; Sung, M.K. Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomized placebo-controlled intervention study. World J. Gastroenterol. 2019, 25, 6129–6144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, X.; Xia, M.; Li, J.; Guo, A.Y.; Zhu, Y.; Yang, X. Quercetin Ameliorates Gut Microbiota Dysbiosis That Drives Hypothalamic Damage and Hepatic Lipogenesis in Monosodium Glutamate-Induced Abdominal Obesity. Front. Nutr. 2021, 8, 671353. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Lushchak, O. Neuroinflammation in pathogenesis of Alzheimer’s disease: Phytochemicals as potential therapeutics. Mech. Ageing Dev. 2020, 189, 111259. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Aggarwal, A.; Kumar, A. Possible nitric oxide mechanism in the protective effect of hesperidin against ischemic reperfusion cerebral injury in rats. Indian J. Exp. Biol. 2011, 49, 609–618. [Google Scholar]
- Naseem, M.; Parvez, S. Hesperidin restores experimentally induced neurotoxicity in Wistar rats. Toxicol. Mech. Methods 2014, 24, 512–519. [Google Scholar] [CrossRef]
- Kamisli, S.; Ciftci, O.; Kaya, K.; Cetin, A.; Kamisli, O.; Ozcan, C. Hesperidin protects brain and sciatic nerve tissues against cisplatin-induced oxidative, histological and electromyographical side effects in rats. Toxicol. Ind. Health 2015, 31, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Kasbe, P.; Pandey, S.N.; Dwivedi, S.; Gurjar, S.S.; Kwatra, M.; Mishra, M.; Venu, A.K.; Sulakhiya, K.; Gogoi, R.; et al. Hesperidin and Silibinin Ameliorate Aluminum-Induced Neurotoxicity: Modulation of Antioxidants and Inflammatory Cytokines Level in Mice Hippocampus. Biol. Trace Elem. Res. 2015, 168, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Parvez, S. Hesperidin ameliorates heavy metal induced toxicity mediated by oxidative stress in brain of Wistar rats. J. Trace Elem. Med. Biol. 2015, 31, 53–60. [Google Scholar] [CrossRef]
- Shagirtha, K.; Bashir, N.; MiltonPrabu, S. Neuroprotective efficacy of hesperetin against cadmium induced oxidative stress in the brain of rats. Toxicol. Ind. Health 2017, 33, 454–468. [Google Scholar] [CrossRef]
- Hemanth Kumar, B.; Dinesh Kumar, B.; Diwan, P.V. Hesperidin, a citrus flavonoid, protects against l-methionine-induced hyperhomocysteinemia by abrogation of oxidative stress, endothelial dysfunction and neurotoxicity in Wistar rats. Pharm. Biol. 2017, 55, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Baradaran, S.; Hajizadeh, M.A.; Ghasemi-Kasman, M. Hesperetin reduces myelin damage and ameliorates glial activation in lysolecithin-induced focal demyelination model of rat optic chiasm. Life Sci. 2018, 207, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, S.; Ghasemi-Kasman, M.; Moghaddam, A.H. Nano-hesperetin enhances the functional recovery and endogenous remyelination of the optic pathway in focal demyelination model. Brain Res. Bull. 2020, 164, 392–399. [Google Scholar] [CrossRef]
- Lee, B.K.; Hyun, S.W.; Jung, Y.S. Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity. Antioxidants 2020, 9, 843. [Google Scholar] [CrossRef]
- Yildiz, M.O.; Celik, H.; Caglayan, C.; Kandemir, F.M.; Gur, C.; Bayav, I.; Genc, A.; Kandemir, O. Neuromodulatory effects of hesperidin against sodium fluoride-induced neurotoxicity in rats: Involvement of neuroinflammation, endoplasmic reticulum stress, apoptosis and autophagy. Neurotoxicology 2022, 90, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Welbat, J.U.; Naewla, S.; Pannangrong, W.; Sirichoat, A.; Aranarochana, A.; Wigmore, P. Neuroprotective effects of hesperidin against methotrexate-induced changes in neurogenesis and oxidative stress in the adult rat. Biochem. Pharmacol. 2020, 178, 114083. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, M.; Kandemir, F.M.; Yildirim, S.; Caglayan, C.; Kucukler, S. Attenuation of sodium arsenite-induced cardiotoxicity and neurotoxicity with the antioxidant, anti-inflammatory, and antiapoptotic effects of hesperidin. Environ. Sci. Pollut. Res. Int. 2021, 28, 10818–10831. [Google Scholar] [CrossRef] [PubMed]
- Noshy, P.A.; Azouz, R.A. Neuroprotective effect of hesperidin against emamectin benzoate-induced neurobehavioral toxicity in rats. Neurotoxicology Teratol. 2021, 86, 106981. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, Y.L.; Luo, L.; Wu, D.M.; Sun, D.X.; Feng, Y.J. Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain. Res. 2006, 171, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Unsal, C.; Kanter, M.; Aktas, C.; Erboga, M. Role of quercetin in cadmium-induced oxidative stress, neuronal damage, and apoptosis in rats. Toxicol. Ind. Health 2015, 31, 1106–1115. [Google Scholar] [CrossRef]
- Sharma, D.R.; Wani, W.Y.; Sunkaria, A.; Kandimalla, R.J.; Sharma, R.K.; Verma, D.; Bal, A.; Gill, K.D. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience 2016, 324, 163–176. [Google Scholar] [CrossRef]
- Dong, F.; Wang, S.; Wang, Y.; Yang, X.; Jiang, J.; Wu, D.; Qu, X.; Fan, H.; Yao, R. Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice. Biochem. Biophys. Res. Commun. 2017, 491, 636–641. [Google Scholar] [CrossRef]
- Al-Otaibi, S.S.; Arafah, M.M.; Sharma, B.; Alhomida, A.S.; Siddiqi, N.J. Synergistic Effect of Quercetin and alpha-Lipoic Acid on Aluminium Chloride Induced Neurotoxicity in Rats. J. Toxicol. 2018, 2018, 2817036. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, X.; Wu, T.; Zhang, W.; Shu, J.; He, Y.; Tang, S.J. Quercetin attenuates AZT-induced neuroinflammation in the CNS. Sci. Rep. 2018, 8, 6194. [Google Scholar] [CrossRef] [Green Version]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-kappaB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146. [Google Scholar] [CrossRef]
- Dora, M.F.; Taha, N.M.; Lebda, M.A.; Hashem, A.E.; Elfeky, M.S.; El-Sayed, Y.S.; Jaouni, S.A.; El-Far, A.H. Quercetin Attenuates Brain Oxidative Alterations Induced by Iron Oxide Nanoparticles in Rats. Int. J. Mol. Sci. 2021, 22, 3829. [Google Scholar] [CrossRef]
- Elblehi, S.S.; Abd El-Maksoud, E.M.; Aldhahrani, A.; Alotaibi, S.S.; Ghamry, H.I.; Elgendy, S.A.; Soliman, M.M.; Shukry, M. Quercetin Abrogates Oxidative Neurotoxicity Induced by Silver Nanoparticles in Wistar Rats. Life 2022, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Kumar, A. Hesperidin pre-treatment attenuates NO-mediated cerebral ischemic reperfusion injury and memory dysfunction. Pharmacol. Rep. 2010, 62, 635–648. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, L.; Liu, M.; Tan, H.; Zheng, L. Hesperidin reduces adverse symptomatic intracerebral hemorrhage by promoting TGF-Î21 for treating ischemic stroke using tissue plasminogen activator. Neurol. Sci. 2020, 41, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Puri, B.K.; Frye, R.E. The putative role of environmental aluminium in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved? Metab. Brain Dis. 2017, 32, 1335–1355. [Google Scholar] [CrossRef] [Green Version]
- Shardlow, E.; Mold, M.; Exley, C. The interaction of aluminium-based adjuvants with THP-1 macrophages in vitro: Implications for cellular survival and systemic translocation. J. Inorg. Biochem. 2020, 203, 110915. [Google Scholar] [CrossRef]
- Exley, C. An aluminium adjuvant in a vaccine is an acute exposure to aluminium. J. Trace Elem. Med. Biol. 2020, 57, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Causality assessment of adverse events following immunization: The problem of multifactorial pathology. F1000Res. 2020, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Siber, G.R. Adjuvants for human vaccines--current status, problems and future prospects. Vaccine 1995, 13, 1263–1276. [Google Scholar] [CrossRef]
- Pellegrino, P.; Clementi, E.; Radice, S. On vaccine’s adjuvants and autoimmunity: Current evidence and future perspectives. Autoimmun. Rev. 2015, 14, 880–888. [Google Scholar] [CrossRef]
- Cirovic, A.; Cirovic, A.; Nikolic, D.; Ivanovski, A.; Ivanovski, P. The adjuvant aluminum fate—Metabolic tale based on the basics of chemistry and biochemistry. J. Trace Elem. Med. Biol. 2021, 68, 126822. [Google Scholar] [CrossRef]
- Luo, Y.; Jin, H.; Guo, Z.N.; Zhang, P.; Zhang, L.Y.; Chen, J.; Yu, Y.; Wang, Y.; Liu, J.; He, Q.Y.; et al. Effect of Hyperhomocysteinemia on Clinical Outcome and Hemorrhagic Transformation After Thrombolysis in Ischemic Stroke Patients. Front. Neurol. 2019, 10, 592. [Google Scholar] [CrossRef] [Green Version]
- Kanter, M.; Unsal, C.; Aktas, C.; Erboga, M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol. Ind. Health 2016, 32, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Xin, Y.; Zheng, K.; Wang, R.; Zhang, X.; Jia, S.; Li, S.; Cao, C.; Zhao, X. Metabolomics analysis of the effects of quercetin on renal toxicity induced by cadmium exposure in rats. Biometals 2021, 34, 33–48. [Google Scholar] [CrossRef]
- Gupta, R.; Shukla, R.K.; Chandravanshi, L.P.; Srivastava, P.; Dhuriya, Y.K.; Shanker, J.; Singh, M.P.; Pant, A.B.; Khanna, V.K. Protective Role of Quercetin in Cadmium-Induced Cholinergic Dysfunctions in Rat Brain by Modulating Mitochondrial Integrity and MAP Kinase Signaling. Mol. Neurobiol. 2017, 54, 4560–4583. [Google Scholar] [CrossRef]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin attenuates neurotoxicity induced by iron oxide nanoparticles. J. Nanobiotechnology 2021, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Magd, N.F.; El-Kashef, D.H.; El-Sherbiny, M.; Eraky, S.M. Hepatoprotective and cognitive-enhancing effects of hesperidin against thioacetamide-induced hepatic encephalopathy in rats. Life Sci. 2023, 313, 121280. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Dhingra, A.K.; Chopra, B.; Guarve, K.; Bhateja, D. Therapeutic Potential and Clinical Evidence of Hesperidin as Neuroprotective Agent. Central Nerv. Syst. Agents Med. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R. Recent Knowledge on Medicinal Plants as Source of Cholinesterase Inhibitors for the Treatment of Dementia. Mini Rev. Med. Chem. 2016, 16, 605–618. [Google Scholar] [CrossRef]
- Grassi, D.; Ferri, C.; Desideri, G. Brain Protection and Cognitive Function: Cocoa Flavonoids as Nutraceuticals. Curr. Pharm. Des. 2016, 22, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Chen, J.Y.; Ouyang, D.; Lu, J.H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, I.E. Cholinesterase Inhibitory Potential of Quercetin towards Alzheimer’s Disease—A Promising Natural Molecule or Fashion of the Day?—A Narrowed Review. Curr. Neuropharmacol. 2021, 19, 2205–2213. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Calfio, C.; Gonzalez, A.; Luttges, V. Novel Nutraceutical Compounds in Alzheimer Prevention. Biomolecules 2022, 12, 249. [Google Scholar] [CrossRef]
- Karthika, C.; Appu, A.P.; Akter, R.; Rahman, M.H.; Tagde, P.; Ashraf, G.M.; Abdel-Daim, M.M.; Hassan, S.S.U.; Abid, A.; Bungau, S. Potential innovation against Alzheimer’s disorder: A tricomponent combination of natural antioxidants (vitamin E, quercetin, and basil oil) and the development of its intranasal delivery. Environ. Sci. Pollut. Res. Int. 2022, 29, 10950–10965. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Spencer, J.P.; Butler, L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Tomata, Y.; Sugiyama, K.; Sugawara, Y.; Tsuji, I. Citrus consumption and incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Br J. Nutr. 2017, 117, 1174–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godos, J.; Caraci, F.; Castellano, S.; Currenti, W.; Galvano, F.; Ferri, R.; Grosso, G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules 2020, 10, 1300. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Dhana, K.; Leurgans, S.E.; Shea, K.; Booth, S.L.; Rajan, K.; Schneider, J.A.; Barnes, L.L. Association of Dietary Intake of Flavonols With Changes in Global Cognition and Several Cognitive Abilities. Neurology 2022. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, L.; Zhu, X.; Wu, W.; Wang, Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zug, C.; Qu, H.; Schluesener, H.; Zhang, Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015, 281, 32–42. [Google Scholar] [CrossRef]
- Javed, H.; Vaibhav, K.; Ahmed, M.E.; Khan, A.; Tabassum, R.; Islam, F.; Safhi, M.M.; Islam, F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J. Neurol. Sci. 2015, 348, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Justin, T.A.; Raja, T.R.; Janakiraman, U.; Manivasagam, T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res. 2015, 40, 767–776. [Google Scholar] [CrossRef]
- Justin, T.A.; William Raja, T.R.; Manivasagam, T.; Janakiraman, U.; Essa, M.M. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2017, 20, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Habibyar, A.F.; Sharma, N.; Khurana, N. PASS assisted prediction and pharmacological evaluation of hesperidin against scopolamine induced amnesia in mice. Eur. J. Pharmacol. 2016, 789, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch. Pharm. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef]
- Kheradmand, E.; Hajizadeh Moghaddam, A.; Zare, M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed. Pharmacother. 2018, 97, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Ishola, I.O.; Jacinta, A.A.; Adeyemi, O.O. Cortico-hippocampal memory enhancing activity of hesperetin on scopolamine-induced amnesia in mice: Role of antioxidant defense system, cholinergic neurotransmission and expression of BDNF. Metab. Brain Dis. 2019, 34, 979–989. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Ibrahim, B.M.M.; Hassan, N.S.; Yousuf, A.F.; Gengaihi, S.E. Exploiting Citrus aurantium seeds and their secondary metabolites in the management of Alzheimer disease. Toxicol. Rep. 2020, 7, 723–729. [Google Scholar] [CrossRef]
- Mandour, D.A.; Bendary, M.A.; Alsemeh, A.E. Histological and imunohistochemical alterations of hippocampus and prefrontal cortex in a rat model of Alzheimer like-disease with a preferential role of the flavonoid “hesperidin”. J. Mol. Histol. 2021, 52, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Aboukhatwa, M.A.; Lei, D.L.; Manaye, K.; Khan, I.; Luo, Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 2010, 58, 911–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richetti, S.K.; Blank, M.; Capiotti, K.M.; Piato, A.L.; Bogo, M.R.; Vianna, M.R.; Bonan, C.D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2011, 217, 10–15. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.T.; Zhou, D.; Bai, X.Y.; Zhou, W.L.; Huang, C.; Song, J.K.; Meng, F.R.; Wu, C.X.; Li, L.; et al. Quercetin protects against the Abeta(25-35)-induced amnesic injury: Involvement of inactivation of rage-mediated pathway and conservation of the NVU. Neuropharmacology 2013, 67, 419–431. [Google Scholar] [CrossRef]
- Regitz, C.; Dussling, L.M.; Wenzel, U. Amyloid-beta (Abeta(1)(-)(4)(2))-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol Nutr. Food Res. 2014, 58, 1931–1940. [Google Scholar] [CrossRef]
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Goncalves Nunes, M.A.; Rubin, M.A.; da Cruz, I.B.; et al. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na(+),K(+)-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guaqueta, A.M.; Munoz-Manco, J.I.; Ramirez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gomez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, M.; Itoh, M.; Ohta, K.; Li, S.; Ueda, M.; Wang, M.X.; Nishida, E.; Islam, S.; Suzuki, C.; Ohzawa, K.; et al. Quercetin reduces eIF2alpha phosphorylation by GADD34 induction. Neurobiol. Aging 2015, 36, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, D.; Rollinger, J.M.; Schmid, A.; Genov, M.; Wohrer, T.; Krenn, L.; Moloney, M.; Kasture, A.; Hummel, T.; Pretsch, A. Prolongation of metallothionein induction combats Ass and alpha-synuclein toxicity in aged transgenic Caenorhabditis elegans. Sci. Rep. 2020, 10, 11707. [Google Scholar] [CrossRef] [PubMed]
- Molaei, A.; Hatami, H.; Dehghan, G.; Sadeghian, R.; Khajehnasiri, N. Synergistic effects of quercetin and regular exercise on the recovery of spatial memory and reduction of parameters of oxidative stress in animal model of Alzheimer’s disease. EXCLI J. 2020, 19, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.M.; Mahmoud, A.A.; Elreedy, H.A.; Ibrahim, K.S.; Ghazy, M.A. Quercetin stimulates the non-amyloidogenic pathway via activation of ADAM10 and ADAM17 gene expression in aluminum chloride-induced Alzheimer’s disease rat model. Life Sci. 2021, 285, 119964. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Miao, Z.; Jiang, X.; Zheng, Y.; Yang, G. Quercitrin improved cognitive impairment through inhibiting inflammation induced by microglia in Alzheimer’s disease mice. Neuroreport 2022, 33, 327–335. [Google Scholar] [CrossRef]
- Baptista, F.I.; Henriques, A.G.; Silva, A.M.; Wiltfang, J.; da Cruz e Silva, O.A. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chem. Neurosci. 2014, 5, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Huebbe, P.; Wagner, A.E.; Boesch-Saadatmandi, C.; Sellmer, F.; Wolffram, S.; Rimbach, G. Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol. Res. 2010, 61, 242–246. [Google Scholar]
- Qu, Z.; Sun, J.; Zhang, W.; Yu, J.; Zhuang, C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radic. Biol. Med. 2020, 159, 87–102. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Q.; Yu, Q. Quercetin enrich diet during the early-middle not middle-late stage of alzheimer’s disease ameliorates cognitive dysfunction. Am. J. Transl. Res. 2018, 10, 1237–1246. [Google Scholar]
- Weng, C.J.; Chen, M.J.; Yeh, C.T.; Yen, G.C. Hepatoprotection of quercetin against oxidative stress by induction of metallothionein expression through activating MAPK and PI3K pathways and enhancing Nrf2 DNA-binding activity. N. Biotechnol. 2011, 28, 767–777. [Google Scholar] [CrossRef]
- Malkki, H. Alzheimer disease: BACE1 inhibition could block CSF tau increase. Nat. Rev. Neurol. 2017, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, N.; Wang, C.; Qin, B.; Zhou, Y.; Xiao, M.; Chang, L.; Yan, L.J.; Zhao, B. Role of RAGE in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2016, 36, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. What is the risk of aluminium as a neurotoxin? Expert. Rev. Neurother. 2014, 14, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Yegambaram, M.; Manivannan, B.; Beach, T.G.; Halden, R.U. Role of environmental contaminants in the etiology of Alzheimer’s disease: A review. Curr. Alzheimer Res. 2015, 12, 116–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Maya, S.; Prakash, T.; Madhu, K.D.; Goli, D. Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed. Pharmacother. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Lai, M.C.; Liu, W.Y.; Liou, S.S.; Liu, I.M. The Citrus Flavonoid Hesperetin Encounters Diabetes-Mediated Alzheimer-Type Neuropathologic Changes through Relieving Advanced Glycation End-Products Inducing Endoplasmic Reticulum Stress. Nutrients 2022, 14, 745. [Google Scholar] [CrossRef]
- Jung, M.; Park, M. Acetylcholinesterase inhibition by flavonoids from Agrimonia pilosa. Molecules 2007, 12, 2130–2139. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Naturforsch. C 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Islam, M.R.; Zaman, A.; Jahan, I.; Chakravorty, R.; Chakraborty, S. In silico QSAR analysis of quercetin reveals its potential as therapeutic drug for Alzheimer’s disease. J. Young Pharm. 2013, 5, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complement. Altern. Med. 2016, 21, NP11-17. [Google Scholar] [CrossRef]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchantchou, F.; Lacor, P.N.; Cao, Z.; Lao, L.; Hou, Y.; Cui, C.; Klein, W.L.; Luo, Y. Stimulation of Neurogenesis and Synaptogenesis by Bilobalide and Quercetin via Common Final Pathway in Hippocampal Neurons. J. Alzheimers. Dis. 2009, 18, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Li, J.; Gao, S.; Yuan, Y.; Sun, Y.; Liu, N.; Li, Y.; Wang, G.; Chen, L.; Shi, J. Network Pharmacology-Based and Experimental Identification of the Effects of Quercetin on Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 589588. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Marzotto, M.; Conforti, A.; Vella, A.; Ortolani, R.; Bellavite, P. Bimodal action of the flavonoid quercetin on basophil function: An investigation of the putative biochemical targets. Clin. Mol. Allergy 2010, 8, 13. [Google Scholar]
- Chirumbolo, S.; Conforti, A.; Ortolani, R.; Vella, A.; Marzotto, M.; Bellavite, P. Stimulus-specific regulation of CD63 and CD203c membrane expression in human basophils by the flavonoid quercetin. Int. Immunopharmacol. 2010, 10, 183–192. [Google Scholar] [CrossRef]
- Zu, G.; Sun, K.; Li, L.; Zu, X.; Han, T.; Huang, H. Mechanism of quercetin therapeutic targets for Alzheimer disease and type 2 diabetes mellitus. Sci. Rep. 2021, 11, 22959. [Google Scholar] [CrossRef]
- Hanaki, M.; Murakami, K.; Gunji, H.; Irie, K. Activity-differential search for amyloid-beta aggregation inhibitors using LC-MS combined with principal component analysis. Bioorg. Med. Chem. Lett. 2022, 61, 128613. [Google Scholar] [CrossRef]
- Liu, C.; Luo, X. Potential molecular and graphene oxide chelators to dissolve amyloid-beta plaques in Alzheimer’s disease: A density functional theory study. J. Mater. Chem. B 2021, 9, 2736–2746. [Google Scholar] [CrossRef]
- Mountaki, C.; Dafnis, I.; Panagopoulou, E.A.; Vasilakopoulou, P.B.; Karvelas, M.; Chiou, A.; Karathanos, V.T.; Chroni, A. Mechanistic insight into the capacity of natural polar phenolic compounds to abolish Alzheimer’s disease-associated pathogenic effects of apoE4 forms. Free Radic. Biol. Med. 2021, 171, 284–301. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In Vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. (Schol. Ed.) 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Yuan, Y.; Fan, S.; Li, L.; Jiang, C.; Mao, C.; Shi, C.; Xu, Y. Peripheral synucleinopathy in Parkinson disease with LRRK2 G2385R variants. Ann. Clin. Transl. Neurol. 2021, 8, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Ni, H.; Li, D.; Gao, R.; Chen, G. The Role of LRRK2 in Neurodegeneration of Parkinson Disease. Curr. Neuropharmacol. 2018, 16, 1348–1357. [Google Scholar] [CrossRef]
- Aalikhani, M.; Safdari, Y.; Jahanshahi, M.; Alikhani, M.; Khalili, M. Comparison Between Hesperidin, Coumarin, and Deferoxamine Iron Chelation and Antioxidant Activity Against Excessive Iron in the Iron Overloaded Mice. Front. Neurosci. 2021, 15, 811080. [Google Scholar] [CrossRef]
- Carradori, S.; Gidaro, M.C.; Petzer, A.; Costa, G.; Guglielmi, P.; Chimenti, P.; Alcaro, S.; Petzer, J.P. Inhibition of Human Monoamine Oxidase: Biological and Molecular Modeling Studies on Selected Natural Flavonoids. J. Agric. Food Chem. 2016, 64, 9004–9011. [Google Scholar] [CrossRef]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yanez, M.; Orallo, F.; Ortuso, F.; Alcaro, S.; et al. A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg. Med. Chem. 2010, 18, 1273–1279. [Google Scholar] [CrossRef]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; Leon, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.H.; Belouahem-Abed, D.; et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine 2018, 40, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Ravaioli, G.; Vafeiadou, K.; Rodriguez-Mateos, A.; Angeloni, C.; Spencer, J.P. Peroxynitrite induced formation of the neurotoxins 5-S-cysteinyl-dopamine and DHBT-1: Implications for Parkinson’s disease and protection by polyphenols. Arch. Biochem. Biophys. 2008, 476, 145–151. [Google Scholar] [CrossRef]
- Vafeiadou, K.; Vauzour, D.; Rodriguez-Mateos, A.; Whiteman, M.; Williams, R.J.; Spencer, J.P. Glial metabolism of quercetin reduces its neurotoxic potential. Arch. Biochem. Biophys. 2008, 478, 195–200. [Google Scholar] [CrossRef]
- Tamilselvam, K.; Braidy, N.; Manivasagam, T.; Essa, M.M.; Prasad, N.R.; Karthikeyan, S.; Thenmozhi, A.J.; Selvaraju, S.; Guillemin, G.J. Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 102741. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.S.; Goes, A.T.; Boeira, S.P.; Prigol, M.; Jesse, C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition 2014, 30, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.S.; Cattelan, S.L.; Ladd, F.V.L.; Ladd, A.A.B.L.; Moreira, A.L.; Bortolotto, V.C.; Silva, M.R.P.; Araújo, S.M.; Prigol, M.; Nogueira, C.W.; et al. Hesperidin Ameliorates Anxiety-Depressive-Like Behavior in 6-OHDA Model of Parkinson’s Disease by Regulating Striatal Cytokine and Neurotrophic Factors Levels and Dopaminergic Innervation Loss in the Striatum of Mice. Mol. Neurobiol. 2020, 57, 3027–3041. [Google Scholar] [CrossRef]
- Antunes, M.S.; Ladd, F.V.L.; Ladd, A.; Moreira, A.L.; Boeira, S.P.; Cattelan Souza, L. Hesperidin protects against behavioral alterations and loss of dopaminergic neurons in 6-OHDA-lesioned mice: The role of mitochondrial dysfunction and apoptosis. Metab. Brain Dis. 2021, 36, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Poetini, M.R.; Araujo, S.M.; Trindade de Paula, M.; Bortolotto, V.C.; Meichtry, L.B.; Polet de Almeida, F.; Jesse, C.R.; Kunz, S.N.; Prigol, M. Hesperidin attenuates iron-induced oxidative damage and dopamine depletion in Drosophila melanogaster model of Parkinson’s disease. Chem. Biol. Interact. 2018, 279, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, L.; Jahanshahi, M.; Jameie, S.B.; Hamid Abadi, H.G.; Nikmahzar, E.; Khalili, M.; Jameie, M.; Jameie, M. 6-OHDA mediated neurotoxicity in SH-SY5Y cellular model of Parkinson disease suppressed by pretreatment with hesperidin through activating L-type calcium channels. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 11–17. [Google Scholar] [CrossRef]
- Kesh, S.; Kannan, R.R.; Sivaji, K.; Balakrishnan, A. Hesperidin downregulates kinases lrrk2 and gsk3beta in a 6-OHDA induced Parkinson’s disease model. Neurosci. Lett. 2021, 740, 135426. [Google Scholar] [CrossRef]
- Ishola, I.O.; Afolayan, O.; Odutola, I.O.; Faniyan, O.; Adeyemi, O.O. Therapeutic potential of hesperidin in Parkinson’s disease with dementia: Inhibition of alpha synuclein and amyloid beta in Drosophila melanogaster. Niger. J. Physiol. Sci. 2021, 36, 43–48. [Google Scholar] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Mitra, N.K.; Kumari, M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci. Lett. 2011, 500, 139–143. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.Y.; Yoon, H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-kappaB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017, 18, 1989. [Google Scholar] [CrossRef] [Green Version]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffari, F.; Hajizadeh Moghaddam, A.; Zare, M. Neuroprotective Effect of Quercetin Nanocrystal in a 6-Hydroxydopamine Model of Parkinson Disease: Biochemical and Behavioral Evidence. Basic Clin. Neurosci. 2018, 9, 317–324. [Google Scholar] [CrossRef]
- Boyina, H.K.; Geethakhrishnan, S.L.; Panuganti, S.; Gangarapu, K.; Devarakonda, K.P.; Bakshi, V.; Guggilla, S.R. In Silico and In Vivo Studies on Quercetin as Potential Anti-Parkinson Agent. Adv. Exp. Med. Biol. 2020, 1195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Madiha, S.; Batool, Z.; Tabassum, S.; Liaquat, L.; Sadir, S.; Shahzad, S.; Naqvi, F.; Saleem, S.; Yousuf, S.; Nawaz, A.; et al. Quercetin exhibits potent antioxidant activity, restores motor and non-motor deficits induced by rotenone toxicity. PLoS ONE 2021, 16, e0258928. [Google Scholar] [CrossRef]
- Wang, W.W.; Han, R.; He, H.J.; Li, J.; Chen, S.Y.; Gu, Y.; Xie, C. Administration of quercetin improves mitochondria quality control and protects the neurons in 6-OHDA-lesioned Parkinson’s disease models. Aging 2021, 13, 11738–11751. [Google Scholar] [CrossRef] [PubMed]
- Josiah, S.S.; Famusiwa, C.D.; Crown, O.O.; Lawal, A.O.; Olaleye, M.T.; Akindahunsi, A.A.; Akinmoladun, A.C. Neuroprotective effects of catechin and quercetin in experimental Parkinsonism through modulation of dopamine metabolism and expression of IL-1beta, TNF-alpha, NF-kappaB, IkappaKB, and p53 genes in male Wistar rats. Neurotoxicology 2022, 90, 158–171. [Google Scholar] [CrossRef]
- Lin, K.J.; Chen, S.D.; Lin, K.L.; Liou, C.W.; Lan, M.Y.; Chuang, Y.C.; Wang, P.W.; Lee, J.J.; Wang, F.S.; Lin, H.Y.; et al. Iron Brain Menace: The Involvement of Ferroptosis in Parkinson Disease. Cells 2022, 11, 3829. [Google Scholar] [CrossRef]
- Ji, Y.; Zheng, K.; Li, S.; Ren, C.; Shen, Y.; Tian, L.; Zhu, H.; Zhou, Z.; Jiang, Y. Insight into the potential role of ferroptosis in neurodegenerative diseases. Front. Cell. Neurosci. 2022, 16, 1005182. [Google Scholar] [CrossRef]
- Shamsi, A.; Shahwan, M.; Khan, M.S.; Husain, F.M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rehman, M.T.; Hassan, M.I.; Islam, A. Elucidating the Interaction of Human Ferritin with Quercetin and Naringenin: Implication of Natural Products in Neurodegenerative Diseases: Molecular Docking and Dynamics Simulation Insight. ACS Omega 2021, 6, 7922–7930. [Google Scholar] [CrossRef]
- Ahn, T.B.; Jeon, B.S. The role of quercetin on the survival of neuron-like PC12 cells and the expression of alpha-synuclein. Neural Regen. Res. 2015, 10, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Quessy, P.; Martinoli, M.G. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell. Mol. Neurobiol. 2009, 29, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Plouffe, M.; Renaud, J.; Provencher, C.; Martinoli, M.G. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxid. Med. Cell. Longev. 2012, 2012, 921941. [Google Scholar] [CrossRef] [Green Version]
- Ballaz, S.; Morales, I.; Rodriguez, M.; Obeso, J.A. Ascorbate prevents cell death from prolonged exposure to glutamate in an in vitro model of human dopaminergic neurons. J. Neurosci. Res. 2013, 91, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Chakraborty, J.; Pakrashi, S.; Sarbajna, A.; Dutta, M.; Bandyopadhyay, J. Quercetin Attenuates Copper-Induced Apoptotic Cell Death and Endoplasmic Reticulum Stress in SH-SY5Y Cells by Autophagic Modulation. Biol. Trace Elem. Res. 2022, 200, 5022–5041. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yu, L.H.; Zhang, J.; Xie, D.J.; Zhang, X.X.; Yu, J.M. Network Pharmacology-Based and Molecular Docking-Based Analysis of Suanzaoren Decoction for the Treatment of Parkinson’s Disease with Sleep Disorder. Biomed. Res. Int. 2021, 2021, 1752570. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Huang, T. Associations of dietary flavonoids and subclasses with total and cardiovascular mortality among 369,827 older people: The NIH-AARP Diet and Health Study. Atherosclerosis 2022, 365, 1–8. [Google Scholar] [CrossRef]
- Fan, X.; Fan, Z.; Yang, Z.; Huang, T.; Tong, Y.; Yang, D.; Mao, X.; Yang, M. Flavonoids-Natural Gifts to Promote Health and Longevity. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Pierpaoli, E.; Giacconi, R.; Costarelli, L.; Piacenza, F.; Basso, A.; Cardelli, M.; Provinciali, M. Pleiotropic Effects of Tocotrienols and Quercetin on Cellular Senescence: Introducing the Perspective of Senolytic Effects of Phytochemicals. Curr. Drug Targets 2016, 17, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, H.; Watchaputi, K.; Hata, J.; Pabuprapap, W.; Suksamrarn, A.; Chua, L.S.; Soontorngun, N. Model yeast as a versatile tool to examine the antioxidant and anti-ageing potential of flavonoids, extracted from medicinal plants. Front. Pharmacol. 2022, 13, 980066. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.; Liu, L.H.B.; Dos Santos, R.V.; Costa, A.F.; Duran, N.; Tasic, L. New Sustainable Process for Hesperidin Isolation and Anti-Ageing Effects of Hesperidin Nanocrystals. Molecules 2020, 25, 4534. [Google Scholar] [CrossRef]
- Gu, Y.; Xue, F.; Xiao, H.; Chen, L.; Zhang, Y. Bamboo Leaf Flavonoids Suppress Oxidative Stress-Induced Senescence of HaCaT Cells and UVB-Induced Photoaging of Mice through p38 MAPK and Autophagy Signaling. Nutrients 2022, 14, 793. [Google Scholar] [CrossRef]
- Zoico, E.; Nori, N.; Darra, E.; Tebon, M.; Rizzatti, V.; Policastro, G.; De Caro, A.; Rossi, A.P.; Fantin, F.; Zamboni, M. Senolytic effects of quercetin in an in vitro model of pre-adipocytes and adipocytes induced senescence. Sci. Rep. 2021, 11, 23237. [Google Scholar] [CrossRef]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 2023. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Kapeta, S.; Chinou, I.; Vassilatou, K.; Papassideri, I.; Gonos, E.S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010, 45, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Du, Y.; Zhang, M.; Zhu, A.R.; Zhang, J. Senolytics Cocktail Dasatinib and Quercetin Alleviate Human Umbilical Vein Endothelial Cell Senescence via the TRAF6-MAPK-NF-kappaB Axis in a YTHDF2-Dependent Manner. Gerontology 2022, 68, 920–934. [Google Scholar] [CrossRef]
- Lewinska, A.; Przybylski, P.; Adamczyk-Grochala, J.; Bloniarz, D.; Litwinienko, G.; Wnuk, M. Senolysis-Based Elimination of Chemotherapy-Induced Senescent Breast Cancer Cells by Quercetin Derivative with Blocked Hydroxy Groups. Cancers 2022, 14, 605. [Google Scholar] [CrossRef]
- Tao, M.; Li, R.; Xu, T.; Zhang, Z.; Wu, T.; Pan, S.; Xu, X. Flavonoids from the mung bean coat promote longevity and fitness in Caenorhabditis elegans. Food Funct. 2021, 12, 8196–8207. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liao, J.; Zhou, H.; Wang, C.C.; Wang, L.; Fan, Y. Flavonoids from Lycium barbarum Leaves Exhibit Anti-Aging Effects through the Redox-Modulation. Molecules 2022, 27, 4952. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xue, J.; Zhang, S.; Zheng, S.; Xue, Y.; Xu, D.; Zhang, X. Composition of peony petal fatty acids and flavonoids and their effect on Caenorhabditis elegans lifespan. Plant Physiol. Biochem. 2020, 155, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shan, J.; Liang, B.; Zhong, L.; Tang, H.; Xu, Y.; Jin, H.; Chen, Y.; Shen, J. Senolytic Drug Quercetin Rescues Survival from Blue Light Toxicity in Drosophila Model. Photochem. Photobiol. 2022. [Google Scholar] [CrossRef]
- Guo, X.; Dong, Z.; Li, Q.; Wan, D.; Zhong, J.; Dongzhi, D.; Huang, M. Flavonoids from Rhododendron nivale Hook. f delay aging via modulation of gut microbiota and glutathione metabolism. Phytomedicine 2022, 104, 154270. [Google Scholar] [CrossRef]
- Zhao, C.P.; Chen, G.Y.; Wang, Y.; Chen, H.; Yu, J.W.; Yang, F.Q. Evaluation of Enzyme Inhibitory Activity of Flavonoids by Polydopamine-Modified Hollow Fiber-Immobilized Xanthine Oxidase. Molecules 2021, 26, 3931. [Google Scholar] [CrossRef]
- Hajizadeh Moghaddam, A.; Shirej Pour, Y.; Mokhtari Sangdehi, S.R.; Hasantabar, V. Evaluation of hesperetin-loaded on multiple wall carbon nanotubes on cerebral ischemia/reperfusion injury in rats. Biomed. Pharmacother. 2021, 138, 111467. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, S.; Mandal, A.K.; Das, N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS ONE 2013, 8, e57735. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-kappaB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.M.; Hoda, M.N.; Raza, S.S.; Khan, M.B.; Javed, H.; Ishrat, T.; Ashafaq, M.; Ahmad, M.E.; Safhi, M.M.; et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem. Res. 2011, 36, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shen, Y.J.; Chen, M.; Zhao, J.Y.; Chen, S.H.; Zhang, W.; Song, J.K.; Li, L.; Du, G.H. Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 2022, 24, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.A.; Blasina, F.; Antúnez, K.; Jones, D.P.; Go, Y.M.; Liang, Y.L.; Dajas, F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Falero, C.; Torres-Rodriguez, S.; Jordan, C.; Licier, R.; Santiago, Y.; Toledo, Z.; Santiago, M.; Serrano, K.; Sosa, J.; Ortiz, J.G. Citrus aurantium increases seizure latency to PTZ induced seizures in zebrafish thru NMDA and mGluR’s I and II. Front. Pharmacol. 2014, 5, 284. [Google Scholar] [CrossRef] [Green Version]
- Oyovwi, M.O.; Ben-Azu, B.; Tesi, E.P.; Oyeleke, A.A.; Uruaka, C.I.; Rotu, R.A.; Aya-Ebi, E.O. Repeated endosulfan exposure induces changes in neurochemicals, decreases ATPase transmembrane ionic-pumps, and increased oxidative/nitrosative stress in the brains of rats: Reversal by quercetin. Pestic. Biochem. Physiol. 2021, 175, 104833. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, H.N.; Kim, C.Y.; Seo, M.D.; Baek, S.H. Synergistic Protection by Isoquercitrin and Quercetin against Glutamate-Induced Oxidative Cell Death in HT22 Cells via Activating Nrf2 and HO-1 Signaling Pathway: Neuroprotective Principles and Mechanisms of Dendropanax morbifera Leaves. Antioxidants 2021, 10, 554. [Google Scholar] [CrossRef]
- Nadar, J.S.; Kale, P.P.; Kadu, P.K.; Prabhavalkar, K.; Dhangar, R. Potentiation of Antidepressant Effects of Agomelatine and Bupropion by Hesperidin in Mice. Neurol Res. Int. 2018, 2018, 9828639. [Google Scholar] [CrossRef] [Green Version]
- Bahramsoltani, R.; Farzaei, M.H.; Farahani, M.S.; Rahimi, R. Phytochemical constituents as future antidepressants: A comprehensive review. Rev. Neurosci. 2015, 26, 699–719. [Google Scholar] [CrossRef]
- Sharma, S.; Raj, K.; Singh, S. Neuroprotective Effect of Quercetin in Combination with Piperine Against Rotenone- and Iron Supplement-Induced Parkinson’s Disease in Experimental Rats. Neurotox. Res. 2020, 37, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, G.B.; Jimenez-Ferrer, E.; Roman-Ramos, R.; Zamilpa, A.; Gonzalez-Cortazar, M.; Leon-Rivera, I.; Vargas-Villa, G.; Herrera-Ruiz, M. A mixture of quercetin 4’-O-rhamnoside and isoquercitrin from Tilia americana var. mexicana and its biotransformation products with antidepressant activity in mice. J. Ethnopharmacol. 2021, 267, 113619. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Rakshit, J.; Bandyopadhyay, J.; Basu, S. Multi-target inhibition ability of neohesperidin dictates its neuroprotective activity: Implication in Alzheimer’s disease therapeutics. Int. J. Biol. Macromol. 2021, 176, 315–324. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, X.; Zhang, Y.; Wang, M.; Liu, T.; Han, J.; Li, J.; Li, Z. Biological evaluation of 7-O-amide hesperetin derivatives as multitarget-directed ligands for the treatment of Alzheimer’s disease. Chem. Biol. Interact. 2021, 334, 109350. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, T.; Qiao, H.; Li, Y.; Xia, M.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Xie, Y.; et al. Research progress of natural products for the treatment of ischemic stroke. J. Integr. Neurosci. 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Chen, S.M.; Chen, X.M.; Mu, R.H.; Wang, S.S.; Geng, D.; Liu, Q.; Yi, L.T. ERK-dependent brain-derived neurotrophic factor regulation by hesperidin in mice exposed to chronic mild stress. Brain Res. Bull. 2016, 124, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, Z.; Fan, S.; Wen, X.; Han, X.; Wang, S.; Wang, Y.; Zhang, Z.; Shan, Q.; Li, M.; et al. Ameliorating effect of quercetin on epilepsy by inhibition of inflammation in glial cells. Exp. Ther. Med. 2020, 20, 854–859. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, G.; Qiu, J.; Yan, W.; Liu, Y.; Ma, Z.; Li, J.; Liu, J.; Shan, N. Quercetin Alleviates Demyelination Through Regulating Microglial Phenotype Transformation to Mitigate Neuropsychiatric Symptoms in Mice with Vascular Dementia. Mol. Neurobiol. 2022, 59, 3140–3158. [Google Scholar] [CrossRef]
- Rasquel-Oliveira, F.S.; Manchope, M.F.; Staurengo-Ferrari, L.; Ferraz, C.R.; Saraiva-Santos, T.; Zaninelli, T.H.; Fattori, V.; Artero, N.A.; Badaro-Garcia, S.; de Freitas, A.; et al. Hesperidin methyl chalcone interacts with NFkB Ser276 and inhibits zymosan-induced joint pain and inflammation, and RAW 264.7 macrophage activation. Inflammopharmacology 2020, 28, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Semis, H.S.; Kandemir, F.M.; Kaynar, O.; Dogan, T.; Arikan, S.M. The protective effects of hesperidin against paclitaxel-induced peripheral neuropathy in rats. Life Sci. 2021, 287, 120104. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Wu, F.; Chi, X.; Pang, Y.; Jin, H.; Sun, Y.; Zhang, S. Neuroprotective Effects of Hesperetin in Regulating Microglia Polarization after Ischemic Stroke by Inhibiting TLR4/NF-kappaB Pathway. J. Healthc. Eng. 2021, 2021, 9938874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, H.; Li, P.; Zhao, H.; Li, R.; Yang, F. Quercetin Administration Following Hypoxia-Induced Neonatal Brain Damage Attenuates Later-Life Seizure Susceptibility and Anxiety-Related Behavior: Modulating Inflammatory Response. Front. Pediatr. 2022, 10, 791815. [Google Scholar] [CrossRef]
- Ruiz-Miyazawa, K.W.; Pinho-Ribeiro, F.A.; Borghi, S.M.; Staurengo-Ferrari, L.; Fattori, V.; Amaral, F.A.; Teixeira, M.M.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q.; et al. Hesperidin Methylchalcone Suppresses Experimental Gout Arthritis in Mice by Inhibiting NF-κB Activation. J. Agric. Food Chem. 2018, 66, 6269–6280. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Ding, Z.; Chen, J.; Chen, T.; Wang, T.; Huang, J. The AMPK-SIRT1-FoxO1-NF-kappaB signaling pathway participates in hesperetin-mediated neuroprotective effects against traumatic brain injury via the NLRP3 inflammasome. Immunopharmacol. Immunotoxicol. 2022, 44, 970–983. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, Y.; Han, N.; He, F.; Li, M.; Bian, Z.; Liu, J.; Sun, T.; Zhu, L. Quercetin suppresses NLRP3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord 2016, 54, 592–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin Attenuates Atherosclerotic Inflammation by Inhibiting Galectin-3-NLRP3 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef]
- Li, H.; Chen, F.J.; Yang, W.L.; Qiao, H.Z.; Zhang, S.J. Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2021, 12, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.A.; El-Magd, M.A.; Ahmed, M.M.; Ghamry, H.I.; Alshahrani, M.Y.; Hegazy, R.A.; Magdy, A.; El-Fotoh, M.F.A. Quercetin alleviated multi-walled carbon nanotubes-induced neurotoxicity in mice through inhibition of oxidation, inflammation, and pyroptosis. Biomed. Pharmacother. 2022, 151, 113160. [Google Scholar] [CrossRef] [PubMed]







| Keywords in Abstracts | Keywords in Titles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Polyphenols | Flavonoids | Hesperidin * | Quercetin | Kaempferol | Apigenin | Naringenin | Taxifolin | Catechin | |
| Neurodegenerative | 261 | 145 | 41 | 146 | 15 | 28 | 29 | 2 | 14 |
| Neuroinflammation | 49 | 54 | 22 | 44 | 11 | 12 | 16 | 1 | 4 |
| Depression | 40 | 53 | 23 | 63 | 2 | 8 | 9 | 0 | 7 |
| Anxiety | 16 | 28 | 16 | 42 | 5 | 4 | 5 | 0 | 4 |
| Cognitive impairment | 32 | 20 | 16 | 42 | 3 | 6 | 7 | 1 | 3 |
| Alzheimer | 167 | 151 | 40 | 100 | 15 | 26 | 26 | 8 | 15 |
| Parkinson | 84 | 60 | 18 | 63 | 11 | 12 | 17 | 1 | 9 |
| Neuroprotective | 181 | 184 | 97 | 312 | 47 | 52 | 60 | 10 | 33 |
| Antidepressant | 16 | 28 | 17 | 29 | 4 | 8 | 6 | 0 | 2 |
| Anxiolytic | 2 | 21 | 8 | 11 | 8 | 5 | 2 | 0 | 0 |
| Models | Treatments | Main Results |
|---|---|---|
| Sleep duration and sedation (mouse) | Valerian extracts containing hesperidin as well as other active ingredients | Increased sleep and sedation [136,137] |
| Anxiety and performance test (hole board test) | Hesperidin low doses | Synergistic effect with diazepam [138,139] |
| Exploration cylinder model (mouse) | Citrus sinensis flowers, hesperidin 11 mg/kg, i.p. | Sedative effect [140] |
| Forced swimming test (mouse) | Byrsonima crassifolia extract 500 mg/kg rich in hesperidin, rutin and quercetin | Antidepressant effect [141] |
| Behavioral disturbances and neurotoxicity in streptozotocin-induced diabetes (rat) | Hesperidin (50 mg/kg) once a day for 4 weeks | Antioxidant and neuroprotective effects [142] |
| Microarray and bioinformatic analysis of the Substantia nigra genome | Valeriana officinalis extract containing hesperidin and linarin | Protection from oxidative stress and neuronal hyperexcitability, hesperidin binding to the K-ATP channel regulatory subunit, the sulfonylurea receptor-1 (SUR1) [143] |
| Models of forced swimming and tail suspension (mouse and rat) | Hesperidin 0.1, 0.3 and 1 mg/kg, i.p.; 50–100 mg/kg, oral, according to different models | Antidepressant effect, modulation of serotonin receptors, inhibition of the L-arginine-NO pathway [144,145,146] |
| Hole board test and plus-maze test | Hesperidin 4–30 mg/kg i.p., 2–100 mg/kg, oral | Antidepressant and anxiolytic-like activity [147] |
| Depression induced by LPS (mouse) | Hesperidin 25, 50, 100 mg/kg | Antidepressant effects, cytokine decrease [148,149] |
| Mild brain trauma, behavioral tests (mouse) | Hesperidin 50 mg/kg for 14 days | Antidepressant effect, inhibition of cytokines and MDA [150] |
| Depression induced by LPS (mouse) | Orange peel extract containing hesperidin 100–200 mg/kg, i.p., 3 days pre-treatment | Significant improvements in behavioral, anorexic and biochemical parameters (oxidation and inflammation markers). Hesperidin binds NF-kappaB [151] |
| Models of forced swimming and suspension on the tail (mouse); PC12 cell line | Hesperidin 100, 200 mg/kg | Antidepressant effect, cytokine inhibition and BDNF stimulation [152] |
| Microglial cells and LPS-treated mouse | Hesperetin 5 mg/kg in vivo, 50–100 μg/mL in vitro | Decreased nitric oxide and cytokines [149,153] |
| Short-term stress, behavioral tests (mouse and rat) | Hesperidin 20, 50 and 100 mg/kg for 14 days | Antidepressant effect. Inhibition of MAO-A activity and decrease in tryptophan hydroxylase-1 expression in the hippocampus [146,154] |
| Containment stress + LPS, behavioral tests (mouse) | Hesperidin 50–100 mg/kg | Anxiolytic and antidepressant effect, reduced oxidative stress [155] |
| Spinal cord injury (rat) | Hesperidin 100 mg/kg | Improved motor dysfunction, decreased cytokines, increased Nrf2/ARE and antioxidant systems [156] |
| Depression in STZ diabetes, open field and elevated maze test (rat) | Hesperidin 50–150 mg/kg | Antidepressant and anxiolytic effects, Nrf2/ARE increase, Protein kinase A increase and other signal transduction mechanisms [157,158,159] |
| STZ diabetes, forced swim test (mouse) | Quercetin 50 and 100 mg/kg, i.p. for 6 weeks | Dose-dependent reduction in the period of immobility, and this effect was comparable to that of fluoxetine (5 mg/kg, i.p.) and imipramine (15 mg/kg, i.p.) [160] |
| Anxiety in mice exposed to the open field measured as body temperature increase | Hypericum perforatum and its components hipericin (0.1 mg/kg), quercitrin (0.6 mg/kg), rutin (1 mg/kg) and quercetin3-O-glucuronide (1.2 mg/kg), pre-treatment 60 min before open field exposure | Anxiolytic-like effect [161] |
| Acute depression from forced swimming test (rat) | Onion powder 50 mg/kg for 14 days | Reduction in immobility time, effects on dopamine metabolism [162] |
| Cytotoxicity of neuronal cells co-cultured with LPS-activated microglia (1 µg/mL) | Quercetin or resveratrol pre-treatment for 24 h at the dose of 0.1 µMol/L | Inhibition of the production of the inflammatory cytokines IL-1alpha and TNF-alpha by microglia. Inhibition of neuronal apoptosis in co-culture [163] |
| Social interaction test, forced swimming (mouse), CRH administration | Quercetin 20–40 mg/kg | Increased time in social interaction and decreased immobility time in forced swimming. Antagonism with CRH and synergism with antalarmine (CRH inhibitor) [164] |
| Depression after olfactory bulbectomy (rat) | Quercetin 40–80 mg/kg for 14 days | Reduction in behavioral alterations, antioxidant and anti-inflammatory effects on microglia, synergism with minocycline [165] |
| Depression after olfactory bulbectomy (rat) | Quercetin 25 mg/kg for 14 days | Improved behavioral testing. Normalization of lipid peroxide (LOOH) levels. Involvement of NMDA receptors and nitric oxide in the pathophysiology of depression [166] |
| Depression in STZ diabetes, open field and elevated maze test (rat) | Quercetin 50 mg/kg for 21 days, i.p. | Improved behavioral tests, no changes in corticosteroid levels [167] |
| Depression induced by 2 h of containment (mouse) | Quercetin 20 mg/kg/mL, i.p. for 15 days before the test | The treatment reversed anxiety and depression; memory performance was improved. Less lipid peroxidation and decreased acetylcholinesterase (AChE), while acetylcholine levels were increased [168] |
| LPS neuroinflammation (intraperitoneal injections for 1 week) (mouse) | Quercetin 30 mg/kg, i.p. for 2 weeks (1 week before LPS, 1 week after LPS) | Inhibition of glia and neuroinflammation in the cortex and hippocampus. Decrease in apoptosis. Improved memory tests [169] |
| Chronic Mild Unpredictable Stress (CUMS), behavioral tests such as forced swimming, tail wagging and open field movement | Quercetin 25 mg/kg after 2 weeks of stress, until 6 weeks. | Improved behavioral tests and markers of oxidative stress and 5-HT levels, decreased glutamate, TNF-alpha and IL-6 levels [43] |
| Psychosocial stress from intrusion (mouse) | Diet enriched with quercetin 0.5–2 g/kg long-term before stress | Improved behavioral tests, decreased astrocyte activation [170] |
| Zebrafish (Danio rerio), behavioral (colony aggregation and placement in the aquarium) and biochemical tests | Quercetin 0.01, 0.1, 1, 10, 100 and 1000 μg/L in the test aquarium | Quercetin at lower concentrations exerted beneficial effects by reducing inflammatory cytokines and oxidative stress. Conversely, when quercetin reached 1000 μg/L, it exerted harmful effects [171] |
| Chronic Mild Unpredictable Stress (CUMS) (mouse) | Quercetin 10, 20, 40 mg/kg/day for 3 weeks | Dose-dependent antidepressant effect (20 and 40 mg). Improved antioxidant indices and Nrf2 in the hippocampus [172] |
| Chronic Mild Unpredictable Stress (CUMS) (mouse) | Quercetin 50 mg/kg/day for 8 weeks | Normalization of metal and trace element levels in serum, reduction in oxidative stress [173] |
| LPS-induced depression (mouse) | Quercitrin single dose 10 mg/kg, i.p. | Antidepressant effect, increase in neuroplasticity signaling molecules, reduction in IL-10, IL-1beta and TNF-alpha in serum, as well as PI3K/AKT/NF-kappaB and MEK/ERK pathways in the hippocampus [174] |
| Cultured microglia cells exposed to LPS (100 ng/mL) for 24 or 48 h | Pre-treatment with quercetin 30–100 μMol/L for 1 h | Reduction in inflammatory cytokines, iNOS and the NLRP3 inflammasome; beneficial effects on mouse models as well [175] |
| Head injury (rat) | Quercetin 5, 20 or 50 mg/kg i.p. at 0.5, 12 and 24 h after trauma | Anti-edema effect, attenuation of cortical inflammatory responses and activation of the Nrf2/HO-1 pathway in the cortex [176] |
| Methamphetamine-induced anxiety (mouse) | Quercetin 50 mg/kg/day for 3 weeks | Antipsychotic, antioxidant and mitochondrial protection activity; reduction in cytokine production by cultured astrocytes [177] |
| Psychosocial stress from intrusion (mouse) | Quercetin 25, 50 and 100 mg/kg, i.p. and ginseng 50 mg/kg, i.p. for 14 days | Improvement of anxiety and depression tests, normalization of the neuroendocrine axis, enhancement of BDNF and inhibition of neuroinflammation [178] |
| LPS neuroinflammation (single injection of 1 mg/kg) in zebrafish, behavioral tests | Quercetin 50–100 mg/kg, i.p. for 7 days | Anxiolytic-like effect, reduction in TNF-alpha and IL-1beta, Lipoperoxides, nitrites and AChE and increase in GSH [179] |
| Models | Treatments | Main Results |
|---|---|---|
| Ischemia by carotid artery occlusion for 30 min followed by reperfusion (rat) | Pre-treatment with hesperidin 50–100 mg kg for 7 days | Improved neurobehavioral alterations, attenuated oxidative damage, restored the activities of antioxidant and mitochondrial enzymes in the brain [204] |
| CCl4 neurotoxicity (rat) | Hesperidin 200 mg/kg for 8 days | Cytoprotective effects, decrease in LPO (lipoperoxides), increase in glutathione peroxidase [205] |
| Cainic acid excitotoxicity (rat) | Hesperidin 10–50 mg/kg, i.p. | Protection of hippocampal cells and decrease in extracellular glutamate [41] |
| Cisplatin neurotoxicity (rat) | Hesperidin 50 mg/kg | Reduction in cellular damage, decrease in lipoperoxidation [206] |
| AlCl3-induced neurotoxicity, memory and maze test (mouse) | Hesperidin 50–100 mg/kg | Reduction in oxidative stress and cognitive impairment [207] |
| Cadmium neurotoxicity (rat) | Hesperidin 40 mg/kg for 21 days | Reduction in changes in oxidative stress biomarkers such as lipoperoxides, AChE, monoamine oxidase, ATPase [208,209] |
| Cognitive impairment from L-methionine-induced hyperhomocysteinemia (rat), maze test. | Hesperidin 100 mg/kg | Improved behavioral testing, abrogation of oxidative stress [210] |
| Lysolecithin demyelination, visual impairment (rats) | Hesperetin 20 mg/kg for 14 or 21 days | Improvement of visual potentials, reduction in microglia, increase in myelin basic protein [211,212] |
| Transient cerebral ischemia induced with middle cerebral artery occlusion (mouse) | Yuzu (a citrus fruit) extract and its active ingredient hesperidin 10 mg/kg | Protection of the blood–brain barrier and claudin-5, reduction in MMP-3/9 (a type of protease) [213] |
| Fluoride toxicity, behavioral and cognition tests (rat) | Hesperidin 100–200 mg/kg for 8 weeks | Neurobehavioral improvement and restoration of brain biochemical changes (AChE and antioxidant activity). Reduction in inflammatory cytokines [32,214] |
| Methotrexate toxicity, biochemical assays (Rat) | Hesperidin 100 mg/kg | Improved levels of BDNF and Nrf2 in the hippocampus and prefrontal cortex [215] |
| Sodium arsenite (NaAsO2)-induced toxicity, biochemical assays (rat) | Hesperidin 100–200 mg/kg for 2 weeks | Antioxidant, anti-inflammatory and antiapoptotic effect [216] |
| Toxicity from emamectin benzoate insecticide, neurobehavioral and cognitive tests (rat) | Hesperidin 100 mg/kg for 8 weeks | Improvement of neural functions and reduction in oxidative stress and inflammation [217] |
| Neurotoxicity from the subcutaneous injection of D-galactose (mouse) | Quercetin 5–10 mg/kg for 8 weeks | Anxiolytic-like effect, increased activity in the open field and learning ability and memory. Increase in SOD, decrease in MDA [218] |
| Cadmium chloride neurotoxicity (rat) | Quercetin 15 mg/kg i.p. for 30 days | Reduction in MDA levels and increase in enzyme antioxidants in the frontal cortex tissue. Reduction in caspase immunoreactivity [219] |
| Aluminum neurotoxicity (rat) | Daily pre-treatment with quercetin 10 mg/kg, intragastric administration | Reduced ROS production, increased SOD activity, Bcl-2 upregulation, structural protection of mitochondria [220] |
| Neurotoxicity from the subcutaneous injection of D-galactose (mouse) | Quercetin 20 or 50 mg/kg for 8 weeks. | Improved memory and behavioral tests, increased expression of Nrf2, HO-1 and SOD [221] |
| Aluminum chloride neurotoxicity (rat) | Quercetin 50 mg/kg and/or alpha-lipoic acid 20 mg/kg i.p. for 2 weeks | Inhibition of lipid peroxidation and normalization of antioxidant enzymes and acetylcholine esterase activity in the brain; synergy with alpha-lipoic acid [222] |
| Zidovudine (azidothymidine, AZT)-induced neurotoxicity and neuroinflammation (mouse) | Quercetin 50 mg/kg for 8 days | Inhibition of inflammatory cytokines produced by astrocytes, mediated by the inhibition of Wnt5a (Wnt Family Member 5A, implicated in chronic inflammatory disorders) [223] |
| Vincristine peripheral neurotoxicity (rat) | Quercetin 25 and 50 mg/kg for 12 days | Increased Nrf2 and HO-1 activities in sciatic nerve tissue, decreased neuronal apoptosis [224] |
| Iron oxide particle neurotoxicity (rat) | Quercetin 25, 50 and 100 mg/kg for 30 days | Dose-dependent decrease in MDA and brain tissue lesions related to neuronal apoptosis [225] |
| Silver nanoparticles (AgNPs)-induced oxidative neurotoxicity (rat) | Quercetin 50 mg/kg for 30 days | Counteracts the pathological effects of AgNPs through the modulation of tight junction proteins, Nrf2 and paraoxonases and the modulation of pro-inflammatory cytokines [226] |
| Models | Treatments | Main Results |
|---|---|---|
| APP/PS1 transgenic mouse | Hesperidin 100 mg/kg a day, 16 weeks | Improvement of cognitive and motor functions, reduction in biochemical disorders [257] |
| APP/PS1 transgenic mouse | Hesperidin 100 mg/kg for 10 days | Improvement of social interactions, anti-inflammatory effects and a reduction in Aβ peptides in the mouse hippocampus and cortex [258] |
| Cognitive impairment from the intracerebroventricular injection of STZ (rat) | Hesperidin 100–200 mg/kg for 15 days | Improved memory, modulation of acetylcholine esterase activity. Improved GSH and the inhibition of NF-kappaB, inducible nitric oxide synthase, cyclooxygenase-2 [259] |
| AD-type lesions after the administration of aluminum (AlCl3) (rat) | Hesperidin 100 mg/kg for 60 days | Attenuation of hippocampal lesions and behavioral disturbances by reducing Aβ(1–40) levels and by inhibiting β- and γ-secretase. Attenuation of acetylcholine esterase [260,261]. |
| Scopolamine-induced AD-type cognitive and memory impairment (mouse) | Hesperidin 100–200 mg/kg for 10 days | Potentiate the therapeutic effect of donepezil (a drug used to counteract the symptoms of dementia) [262] |
| APP/PS1 transgenic mouse | Hesperidin 40 mg/kg for 90 days | Attenuation of cognitive impairment, increase in Nrf2/ARE and antioxidant enzymes, deactivation of the receptor of the advanced glycation end products (RAGE)-mediated pathway [263] |
| AD-like cognitive and memory impairment from the intracerebroventricular injection of STZ (rat) | Hesperetin and nano-hesperetin 10, 20 mg/kg for 3 weeks | Improvement in symptoms, increased activity of antioxidant enzymes and GSH levels and decreased malondialdehyde in the hippocampus [264] |
| AD-type lesions after the administration of aluminum (AlCl3) (rat) | Hesperidin 100 mg/kg for 60 days | Behavioral improvements, reduction in the phosphorylation of the tau protein, markers of inflammation, nitric oxide and apoptosis [71] |
| Aβ(1–42) injection-induced brain degeneration (mouse) | Hesperetin 50 mg/kg for 6 weeks | Attenuated oxidative stress and inflammation, as assessed by Nrf2, ROS and NF-kappaB expression in the hippocampus, cortex and HT22 cells in vitro [70] |
| Scopolamine-induced AD-type cognitive and memory impairment (mouse) | Hesperetin 1, 5 or 50 mg/kg for 3 days | Improved non-spatial and spatial learning and reduced memory impairment [265] |
| AD-type lesions after the administration of aluminum (AlCl3) (rat) | Hesperidin 125–250 mg/kg for 2 weeks | Protection against cognitive impairment, reduction in acetylcholine esterase and Aβ levels [266] |
| Scopolamine-induced AD-type cognitive and memory impairment (rat) | Hesperidin 100 mg/kg for 28 days | Reduction in spatial memory deficits, redox imbalance, Aβ(1–42) and AChE [267] |
| APP/PS1 transgenic mouse | Quercetin 50 mg/kg for 4 months | Increased BDNF levels and decreased Aβ in the hippocampus, cognitive improvement [268] |
| Scopolamine-induced amnesia (zebrafish) | Pre-treatment with quercetin or rutin 50 mg/kg, i.p. | Protection in memory and behavioral tests [269] |
| Amnesia induced by the intracerebral injection of Aβ(25–35) (mouse) | Quercetin 10–40 mg/kg for 8 days after Aβ injection | Improved learning and memory abilities, decreased neurovascular oxidation, improved cholinergic activity, inactivation of the RAGE-mediated pathway [270] |
| Motor paralysis in the human Aβ-producing transgenic strain of the Caenorhabditis elegans nematode | Quercetin doses >10 µMol/L for 48 h. | Dose-dependent decrease in aggregated Aβ and related paralysis [271] |
| APP/PS1 transgenic mouse | Quercetin 50 mg/kg for 16 weeks | Reduction in learning deficits, reduction in scattered senile plaques and improvement of mitochondrial dysfunction [272] |
| Memory impairment induced by cadmium exposure (rat) | Quercetin 5, 25 or 50 mg/kg for 45 days | Protection of AChE and Na(+),K(+)-ATPase, reduction in oxidative stress markers [273] |
| Elderly triple transgenic AD model mice | Quercetin 25 mg/kg i.p. every 48 h for 3 months | Decreased beta-amyloidosis, tauopathy, astrogliosis and microgliosis in the hippocampus and amygdala. Decreased BACE1-mediated APP cleavage. Improved performance in learning and memory [274] |
| APP23 transgenic mouse (human amyloid precursor protein overexpression) | Long-term diet with 20% casein and 0.5% quercetin | Reduction in presenilin 1 expression and Aβ secretion, prevention of mental deterioration [275] |
| Motor paralysis in the human Aβ-producing transgenic strain of the Caenorhabditis elegans nematode | Quercetin 33 µMol/L for 48 h. | Reduction in symptoms of paralysis, stimulation of metallothionein and lengthening of the lifespan [276] |
| STZ-induced memory impairment (rat) | Quercetin 80 mg/kg i.p., for 3 weeks, plus regular exercise for 60 days | Quercetin and exercise synergistically improved spatial memory and reduced oxidative stress [277] |
| AD induced by aluminum chloride (AlCl3) for 28 days. | Quercetin 25–50 mg/kg for 28 days after induction | Attenuation of behavioral deficits, reduction in insoluble Aβ plaques in the hippocampus, increased activity of metalloproteases [278] |
| 5XFAD transgenic mouse expressing human APP and PSEN1 with a total of five AD-linked mutations | Diet enriched with quercitrin 50–100 mg/kg for 3 months | Improved behavioral testing and reduction in amyloid plaques, inhibition of microglial cytokines [279] |
| Models | Treatments | Main Results |
|---|---|---|
| Cortical neurons treated with 5-S-cysteinyl-dopamine in vitro | Various polyphenols including hesperetin and quercetin 0.1–3.0 μMol/L | Protection from neuronal damage [316,317] |
| Rotenone-induced apoptosis in human neuroblastoma SK-N-SH cells in vitro | Hesperidin 4–30 nMol/L | Preservation of mitochondrial function, inhibition of ROS and the molecular mechanisms of apoptosis (Bax, cytochrome c and caspases 3 and 9, and the downregulation of Bcl-2) [318]. |
| 6-Hydroxydopamine (6-OHDA)-induced PD (elderly mouse) | Hesperidin 50 mg/kg for 28 days | Protection of glutathione peroxidase and catalase activity, striatum dopamine levels, improvement of cognitive symptoms and modulation of proinflammatory cytokines [319,320,321] |
| PD induced by iron (Fe) intake in Drosophila melanogaster (fruit fly) | Diet with hesperidin 10 µMol/L | Decreased Fe concentration in the head, normalization of dopamine levels and cholinergic activity and improvement of motor function impaired by Fe; inhibition of oxidative stress and mitochondrial protection [322] |
| Model of PD on 6-OHDA-intoxicated SH-SY5Y neuroblastoma cells | Hesperidin 1–10 µMol/L | Preservation of cell vitality, rebalancing of Ca2+ homeostasis, reduction in oxidative stress [323,324] |
| PD and AD induced in Drosophila melanogaster with the UAS-GAL4 system | Hesperidin 1, 5 or 10 mMol/L in food throughout life | Protection from the development of the disease and symptoms [325] |
| Iron dextran-induced PD (mouse) | Hesperidin or Coumarin 50–100 mg/kg and Desferal 25 mg/kg 4 times/week for 4 weeks | Hesperidin chelates iron, like Desferal [312] |
| PD induced by 6-OHDA (rat) | Quercetin 30 mg/kg for 14 days | Increased striatal dopamine, antioxidant enzymes and neuronal survival [326] |
| Neurotoxicity due to manganese excess (500 μMol/L Mn for 24 h) on cultured SK-N-MC neurons | Quercetin 10–20 μg/mL | Protective effect of quercetin on cell mortality, decreased NF-kappaB but increased heme oxygenase-1 (HO-1) and Nrf2 [327] |
| Neurotoxicity due to manganese excess (MnCl2 15 mg/kg i.p. for 8 days) (rat) | Quercetin 25–50 mg/kg for 8 days | Decreased oxidative stress, decreased various apoptotic markers and upregulated anti-apoptotic Bcl-2 proteins and SOD [327] |
| MitoPark mouse model with the inactivation of mitochondrial transcription factor A in dopaminergic neurons | Quercetin 25 mg/kg for 6 weeks | Counteraction of behavioral deficits, striatal dopamine depletion and neuronal cell loss. Activation of two cell survival kinases and BDNF, increased mitochondrial bioenergetic capacity [328] |
| PD induced by 6-OHDA (rat) | Quercetin 10 and 25 mg/kg (in normal form or in nanocrystals) for 4 weeks | Memory preservation, increase in antioxidant enzymes and total glutathione. Higher efficacy in the form of nanocrystals [329] |
| Parkinsonism induced by the administration of rotenone at a dose of 1.5 mg/kg for 28 days (rat) | Quercetin, 15–50 mg/kg, co-administered with rotenone | Dose-dependent neuroprotective effects by improving behavioral tests and signs of oxidative stress. Drug targets could be aromatic L-amino acid decarboxylase and catechol-O-methyltransferase [330]. |
| Parkinsonism induced by the administration of rotenone at a dose of 1.5 mg/kg in rats for 8 days (rat) | Quercetin, 50 mg/kg for 2 weeks before and after rotenone | Restoration of motor and cognitive functions, improvement of antioxidant enzymes in the brain [331] |
| PD induced by 6-OHDA in vitro on PC12 cells and in vivo (rat). | Quercetin 20 μMol/L in vitro and 30 mg/kg for 14 days in vivo | Improved mitochondrial function, reduced oxidative stress, increased levels of PINK1 and Parkin markers and decreased α-synuclein protein expression in PC12 cells. Reduction in PD-like behaviors and α-synuclein accumulation in rats [332] |
| Parkinsonism induced by the multiple-dose administration of rotenone at a dose of 1.5 mg/kg (rat) | Quercetin 5–20 mg/kg subcutaneously for 3 days | Attenuation of neurobehavioral deficits caused by rotenone. Attenuation of striatal redox stress and neurochemical dysfunction of the NF-kappaB and IkappaKB genes [333] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. https://doi.org/10.3390/antiox12020280
Bellavite P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants. 2023; 12(2):280. https://doi.org/10.3390/antiox12020280
Chicago/Turabian StyleBellavite, Paolo. 2023. "Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action" Antioxidants 12, no. 2: 280. https://doi.org/10.3390/antiox12020280
APA StyleBellavite, P. (2023). Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants, 12(2), 280. https://doi.org/10.3390/antiox12020280