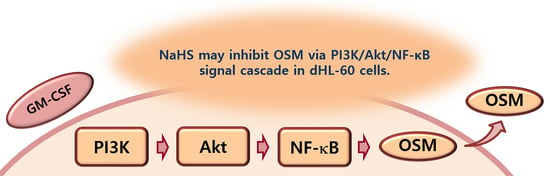
Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability
2.4. OSM Measurement
2.5. Real-Time Quantitative PCR
2.6. Western Blot Analysis
2.7. Immunofluorescence Analysis
2.8. Statistical Analysis
3. Results
3.1. NaHS Represses OSM Secretion in dHL-60 Cells
3.2. NaHS Decreases OSM mRNA Expression in dHL-60 Cells
3.3. NaHS Inhibits PI3K Phosphorylation in dHL-60 Cells
3.4. NaHS Suppresses Akt Phosphorylation in dHL-60 Cells
3.5. NaHS Downregulates NF-κB Phosphorylation in dHL-60 Cells
3.6. NaHS Decreases p-NF-κB and OSM Immunofluorescence Staining in dHL-60 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Sodium Thiosulfate Improves Hypertension in Rats with Adenine-Induced Chronic Kidney Disease. Antioxidants 2022, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Ramil, M.; Burguera, E.F.; Hermida-Gómez, T.; Caramés, B.; Oreiro-Villar, N.; Meijide-Faílde, R.; Blanco, F.J.; Vaamonde-García, C. Reduced Levels of H2S in Diabetes-Associated Osteoarthritis Are Linked to Hyperglycaemia, Nrf-2/HO-1 Signalling Downregulation and Chondrocyte Dysfunction. Antioxidants 2022, 11, 628. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Rodríguez, L.; Bai, X.; Batallé, G.; Roch, G.; Pouso-Vázquez, E.; Balboni, G.; Pol, O. Hydrogen Sulfide Inhibits Inflammatory Pain and Enhances the Analgesic Properties of Delta Opioid Receptors. Antioxidants 2021, 10, 1977. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.S.; Zhang, Y.; Wang, T.Y.; Li, X.; Ma, C.; Xu, Z.M.; Sun, Q.; Xu, X.; Chen, G. Therapeutic applications of hydrogen sulfide and novel donors for cerebral ischemic stroke: A narrative review. Med. Gas Res. 2023, 13, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, X.; Dang, B.; Wu, F.; Gou, K.; Wang, C.; Lin, C. Hydrogen sulfide protects retina from blue light-induced photodamage and degeneration via inhibiting ROS-mediated ER stress-CHOP apoptosis signal. Redox Rep. 2022, 27, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, A.; Micheli, L.; Mannelli, L.D.C.; Ghelardini, C.; Gratteri, P.; Nocentini, A.; Supuran, C.T. Development of Hydrogen Sulfide-Releasing Carbonic Anhydrases IX- and XII-Selective Inhibitors with Enhanced Antihyperalgesic Action in a Rat Model of Arthritis. J. Med. Chem. 2022, 65, 13143–13157. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Jeong, H.J.; Kim, H.M. Hydrogen sulfide diminishes the levels of thymic stromal lymphopoietin in activated mast cells. Arch. Dermatol. Res. 2016, 308, 103–113. [Google Scholar] [CrossRef]
- Kim, N.R.; Nam, S.Y.; Ryu, K.J.; Kim, H.M.; Jeong, H.J. Effects of bamboo salt and its component, hydrogen sulfide, on enhancing immunity. Mol. Med. Rep. 2016, 14, 1673–1680. [Google Scholar] [CrossRef]
- Zarling, J.M.; Shoyab, M.; Marquardt, H.; Hanson, M.B.; Lioubin, M.N.; Todaro, G.J. Oncostatin M: A growth regulator produced by differentiated histiocytic lymphoma cells. Proc. Natl. Acad. Sci. USA 1986, 83, 9739–9743. [Google Scholar] [CrossRef]
- Yang, X.; Shao, C.; Duan, L.; Hou, X.; Huang, Y.; Gao, L.; Zong, C.; Liu, W.; Jiang, J.; Ye, F.; et al. Oncostatin M promotes hepatic progenitor cell activation and hepatocarcinogenesis via macrophage-derived tumor necrosis factor-α. Cancer Lett. 2021, 517, 46–54. [Google Scholar] [CrossRef]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Görtz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef]
- Reid, J.; Zamuner, S.; Edwards, K.; Rumley, S.A.; Nevin, K.; Feeney, M.; Zecchin, C.; Fernando, D.; Wisniacki, N. In vivo affinity and target engagement in skin and blood in a first-time-in-human study of an anti-oncostatin M monoclonal antibody. Br. J. Clin. Pharmacol. 2018, 84, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lei, L.; Hu, J.; Li, Y. Oncostatin M upregulates Livin to promote keratinocyte proliferation and survival via ERK and STAT3 signalling pathways. Exp. Physiol. 2020, 105, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Asif, M.; Singh, V.; Dubey, P.; Malik, S.A.; Lone, M.U.; Tewari, B.N.; Baghel, K.S.; Pal, S.; Nagar, G.K.; et al. M2 polarization of macrophages by Oncostatin M in hypoxic tumor microenvironment is mediated by mTORC2 and promotes tumor growth and metastasis. Cytokine 2019, 118, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, Y.; Liu, H.; Yang, H.; Lu, P.; Shi, Y.; Wang, X.; Zheng, W.; Yu, X.; Xu, Y.; et al. Oncostatin M sensitizes keratinocytes to UVB-induced inflammation via GSDME-mediated pyroptosis. J. Dermatol. Sci. 2021, 104, 95–103. [Google Scholar] [CrossRef]
- Zoaiter, M.; Nasser, R.; Hage-Sleiman, R.; Abdel-Sater, F.; Badran, B.; Zeaiter, Z. Helicobacter pylori outer membrane vesicles induce expression and secretion of oncostatin M in AGS gastric cancer cells. Braz. J. Microbiol. 2021, 52, 1057–1066. [Google Scholar] [CrossRef]
- Mashimo, K.; Usui-Ouchi, A.; Ito, Y.; Wakasa-Arai, R.; Yokoi, N.; Kawasaki, S.; Murakami, A.; Matsuda, A.; Ebihara, N. Role of oncostatin M in the pathogenesis of vernal keratoconjunctivitis: Focus on tissue remodeling. Jpn. J. Ophthalmol. 2021, 65, 144–153. [Google Scholar] [CrossRef]
- Kubin, T.; Pöling, J.; Kostin, S.; Gajawada, P.; Hein, S.; Rees, W.; Wietelmann, A.; Tanaka, M.; Lörchner, H.; Schimanski, S.; et al. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 2011, 9, 420–432. [Google Scholar] [CrossRef]
- Stephens, J.M.; Elks, C.M. Oncostatin M: Potential Implications for Malignancy and Metabolism. Curr. Pharm. Des. 2017, 23, 3645–3657. [Google Scholar] [CrossRef]
- Garcia, J.P.; Utomo, L.; Rudnik-Jansen, I.; Du, J.; Zuithoff, N.; Krouwels, A.; van Osch, G.; Creemers, L.B. Association between Oncostatin M Expression and Inflammatory Phenotype in Experimental Arthritis Models and Osteoarthritis Patients. Cells 2021, 10, 508. [Google Scholar] [CrossRef]
- Kang, H.J.; Kang, J.S.; Lee, S.H.; Hwang, S.J.; Chae, S.W.; Woo, J.S.; Lee, H.M. Upregulation of oncostatin m in allergic rhinitis. Laryngoscope 2005, 115, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Pothoven, K.L.; Norton, J.E.; Suh, L.A.; Carter, R.G.; Harris, K.E.; Biyasheva, A.; Welch, K.; Shintani-Smith, S.; Conley, D.B.; Liu, M.C.; et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J. Allergy Clin. Immunol. 2017, 139, 1966–1978.e9. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Ko, S.G.; Park, H.J.; Moon, P.D. Dexamethasone Attenuates Oncostatin M Production Via Suppressing of PI3K/Akt/NF-κB Signaling in Neutrophil-Like Differentiated HL-60 Cells. Molecules 2022, 27, 129. [Google Scholar] [CrossRef]
- Han, W.; Xiong, Y.; Li, Y.; Fang, W.; Ma, Y.; Liu, L.; Li, F.; Zhu, X. Anti-arthritic effects of clematichinenoside (AR-6) on PI3K/Akt signaling pathway and TNF-α associated with collagen-induced arthritis. Pharm. Biol. 2013, 51, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Tsai, C.H.; Fong, Y.C.; Huang, Y.L.; Wang, S.J.; Chang, Y.S.; Tang, C.H. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. Int. J. Mol. Sci. 2014, 15, 15778–15790. [Google Scholar] [CrossRef]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef]
- Lv, B.; Deng, L.; Xie, T.; Wei, X.; Liu, X.; Tan, W.; Wang, X.; Gao, X. Evaluation of the anti-inflammatory and antioxidant pharmcodynamic compoents of naoxintong capsules as a basis of broad spectrum effects. Pharm. Biol. 2021, 59, 242–251. [Google Scholar] [CrossRef]
- Su, C.M.; Lee, W.L.; Hsu, C.J.; Lu, T.T.; Wang, L.H.; Xu, G.H.; Tang, C.H. Adiponectin Induces Oncostatin M Expression in Osteoblasts through the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2016, 17, 29. [Google Scholar] [CrossRef]
- Elbjeirami, W.M.; Donnachie, E.M.; Burns, A.R.; Smith, C.W. Endothelium-derived GM-CSF influences expression of oncostatin M. Am. J. Physiol. Cell Physiol. 2011, 301, C947–C953. [Google Scholar] [CrossRef]
- Moon, P.D.; Lee, J.S.; Kim, H.Y.; Han, N.R.; Kang, I.; Kim, H.M.; Jeong, H.J. Heat-treated Lactobacillus plantarum increases the immune responses through activation of natural killer cells and macrophages on in vivo and in vitro models. J. Med. Microbiol. 2019, 68, 467–474. [Google Scholar] [CrossRef]
- Han, N.R.; Ko, S.G.; Moon, P.D.; Park, H.J. Chloroquine attenuates thymic stromal lymphopoietin production via suppressing caspase-1 signaling in mast cells. Biomed. Pharmacother. 2021, 141, 111835. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Paudel, S.; Pandey, A.; Yadav, P.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.H.; Khalilullah, H.; Verma, A. Guava Leaf Essential Oil as a Potent Antioxidant and Anticancer Agent: Validated through Experimental and Computational Study. Antioxidants 2022, 11, 2204. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.; Olivares, A.; Andreu-Gálvez, M.; Achel, D.G.; Mercado, A.M.; Alcaraz-Saura, M. Paradoxical Radiosensitizing Effect of Carnosic Acid on B16F10 Metastatic Melanoma Cells: A New Treatment Strategy. Antioxidants 2022, 11, 2166. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.Y.; Kareem, S.M.; Atef, A.; Safwat, N.A.; Shehata, R.M.; Yosri, M.; Youssef, M.; Baakdah, M.M.; Sami, R.; Baty, R.S.; et al. Optimization of Supercritical Carbon Dioxide Extraction of Saussurea costus Oil and Its Antimicrobial, Antioxidant, and Anticancer Activities. Antioxidants 2022, 11, 1960. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Uzair, M.; Ali, S.; Qamar, M.; Ahmad, N.; Abbas, M.W.; Esatbeyoglu, T. Dryopteris juxtapostia Root and Shoot: Determination of Phytochemicals; Antioxidant, Anti-Inflammatory, and Hepatoprotective Effects; and Toxicity Assessment. Antioxidants 2022, 11, 1670. [Google Scholar] [CrossRef]
- Moon, P.D.; Han, N.R.; Kim, H.M.; Jeong, H.J. High-Fat Diet Exacerbates Dermatitis through Up-Regulation of TSLP. J. Investig. Dermatol. 2019, 139, 1198–1201. [Google Scholar] [CrossRef]
- Han, N.R.; Ko, S.G.; Moon, P.D.; Park, H.J. Ginsenoside Rg3 attenuates skin disorders via down-regulation of MDM2/HIF1α signaling pathway. J. Ginseng Res. 2021, 45, 610–616. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, H.J.; Lee, J.S.; Kim, H.Y.; Moon, P.D.; Kim, H.M.; Jeong, H.J. The immune-enhancing effect of anthocyanin-fucoidan nanocomplex in RAW264.7 macrophages and cyclophosphamide-induced immunosuppressed mice. J. Food Biochem. 2021, 45, e13631. [Google Scholar] [CrossRef]
- Moon, P.D.; Han, N.R.; Lee, J.S.; Kim, H.M.; Jeong, H.J. p-coumaric acid, an active ingredient of Panax ginseng, ameliolates atopic dermatitis-like skin lesions through inhibition of thymic stromal lymphopoietin in mice. J. Ginseng Res. 2021, 45, 176–182. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, H.Y.; Kang, S.; Kim, M.H.; Yoon, K.W.; Moon, P.D.; Kim, H.M.; Jeong, H.J. Chrysophanol, an anthraquinone from AST2017-01, possesses the anti-proliferative effect through increasing p53 protein levels in human mast cells. Inflamm. Res. 2019, 68, 569–579. [Google Scholar] [CrossRef]
- Han, N.R.; Moon, P.D.; Kim, H.M.; Jeong, H.J. TSLP Exacerbates Septic Inflammation via Murine Double Minute 2 (MDM2) Signaling Pathway. J. Clin. Med. 2019, 8, 1350. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Han, N.R.; Lee, J.S.; Kim, H.M.; Jeong, H.J. Ursolic acid downregulates thymic stromal lymphopoietin through the blockade of intracellular calcium/caspase-1/NF-κB signaling cascade in HMC-1 cells. Int. J. Mol. Med. 2019, 43, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Han, N.R.; Lee, J.S.; Hong, S.; Yoo, M.S.; Kim, H.J.; Kim, J.H.; Kang, S.; Jee, H.W.; Kim, H.M.; et al. Use of Physcion to Improve Atopic Dermatitis-Like Skin Lesions through Blocking of Thymic Stromal Lymphopoietin. Molecules 2019, 24, 1484. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Han, S.J.; Moon, P.D.; Hong, S.; Kim, H.; Li, Y.H.; Kim, H.M.; Jeong, H.J. Effect of dexamethasone injection into Zusanli (ST 36) acupoint on ovalbumin-induced allergic rhinitis. J. Tradit. Chin. Med. 2019, 39, 307–314. [Google Scholar]
- Li, X.; Khan, D.; Rana, M.; Hänggi, D.; Muhammad, S. Doxycycline Attenuated Ethanol-Induced Inflammaging in Endothelial Cells: Implications in Alcohol-Mediated Vascular Diseases. Antioxidants 2022, 11, 2413. [Google Scholar] [CrossRef]
- Wang, B.; Cui, S.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Tang, X.; Chen, W. Cyanidin Alleviated CCl4-Induced Acute Liver Injury by Regulating the Nrf2 and NF-κB Signaling Pathways. Antioxidants 2022, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chang, T.M.; Huang, H.C. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Attenuate Mast Cell Activation. Antioxidants 2022, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein Decreases Inflammation and Oxidative Stress and Prevents Iron Accumulation and Lipid Peroxidation at Glutamate-Induced Neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.D.; Kim, M.H.; Lim, H.S.; Oh, H.A.; Nam, S.Y.; Han, N.R.; Kim, M.J.; Jeong, H.J.; Kim, H.M. Taurine, a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. BioFactors 2015, 41, 190–197. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, K.C.; Kim, J.S.; Ko, S.G.; Park, H.J.; Moon, P.D. The immune-enhancing effects of a mixture of Astragalus membranaceus (Fisch.) Bunge, Angelica gigas Nakai, and Trichosanthes Kirilowii (Maxim.) or its active constituent nodakenin. J. Ethnopharmacol. 2022, 285, 114893. [Google Scholar] [CrossRef]
- Pothoven, K.L.; Norton, J.E.; Hulse, K.E.; Suh, L.A.; Carter, R.G.; Rocci, E.; Harris, K.E.; Shintani-Smith, S.; Conley, D.B.; Chandra, R.K.; et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J. Allergy Clin. Immunol. 2015, 136, 737–746.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, J.L.; Baines, K.J.; Boyle, M.J.; Scott, R.J.; Gibson, P.G. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Exp. Lung Res. 2009, 35, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Streiff, R.J.; Liu, J.; Spence, M.J.; Vestal, R.E. Cloning and characterization of human oncostatin M promoter. Nucleic Acids Res. 1999, 27, 4649–4657. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.; Edwards, S.W.; Bucknall, R.C.; Moots, R.J. Secretion of oncostatin M by neutrophils in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 1430–1436. [Google Scholar] [CrossRef]
- Grenier, A.; Dehoux, M.; Boutten, A.; Arce-Vicioso, M.; Durand, G.; Gougerot-Pocidalo, M.A.; Chollet-Martin, S. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 1999, 93, 1413–1421. [Google Scholar] [CrossRef]
- Mozaffarian, A.; Brewer, A.W.; Trueblood, E.S.; Luzina, I.G.; Todd, N.W.; Atamas, S.P.; Arnett, H.A. Mechanisms of oncostatin M-induced pulmonary inflammation and fibrosis. J. Immunol. 2008, 181, 7243–7253. [Google Scholar] [CrossRef]
- Modur, V.; Feldhaus, M.J.; Weyrich, A.S.; Jicha, D.L.; Prescott, S.M.; Zimmerman, G.A.; McIntyre, T.M. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. J. Clin. Investig. 1997, 100, 158–168. [Google Scholar] [CrossRef]
- Botelho, F.; Dubey, A.; Ayaub, E.A.; Park, R.; Yip, A.; Humbles, A.; Kolbeck, R.; Richards, C.D. IL-33 Mediates Lung Inflammation by the IL-6-Type Cytokine Oncostatin, M. Mediat. Inflamm. 2020, 2020, 4087315. [Google Scholar] [CrossRef]
- Moon, P.D.; Kim, H.M. Anti-inflammatory effect of phenethyl isothiocyanate, an active ingredient of Raphanus sativus Linne. Food Chem. 2012, 131, 1332–1339. [Google Scholar] [CrossRef]
- Moon, P.D.; Lee, B.H.; Jeong, H.J.; An, H.J.; Park, S.J.; Kim, H.R.; Ko, S.G.; Um, J.Y.; Hong, S.H.; Kim, H.M. Use of scopoletin to inhibit the production of inflammatory cytokines through inhibition of the IkappaB/NF-kappaB signal cascade in the human mast cell line HMC-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef]
- Moon, P.D.; Kim, H.M. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-κB pathway in mast cells. Cytokine 2011, 54, 239–243. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fu, Y.; Yan, B.; Tan, H.; Li, H.; Li, J.; Huang, D.; Huang, Z.; Lai, J.; Feng, H.; et al. Curcumol Alleviates the Inflammation of Nucleus Pulposus Cells via the PI3K/Akt/NF-κB Signaling Pathway and Delays Intervertebral Disk Degeneration. World Neurosurg. 2021, 155, e402–e411. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, X.; Li, S. Propofol promotes migration, alleviates inflammation, and apoptosis of lipopolysaccharide-induced human pulmonary microvascular endothelial cells by activating PI3K/AKT signaling pathway via upregulating APOM expression. Drug Dev. Res. 2022, 83, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.G.; Song, C.H.; Yi, H.K.; Hwang, P.H.; Kim, J.S.; Lee, K.S.; Lee, Y.C. Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma. J. Clin. Investig. 2003, 111, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, H.K.; Hayflick, J.S.; Lee, Y.C.; Puri, K.D. Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J. 2006, 20, 455–465. [Google Scholar] [CrossRef]
- Bao, Z.; Zhang, P.; Yao, Y.; Lu, G.; Tong, Z.; Yan, B.; Tu, L.; Yang, G.; Zhou, J. Deguelin Attenuates Allergic Airway Inflammation via Inhibition of NF-κb Pathway in Mice. Int. J. Biol. Sci. 2017, 13, 492–504. [Google Scholar] [CrossRef]
- El-Hashim, A.Z.; Renno, W.M.; Abduo, H.T.; Jaffal, S.M.; Akhtar, S.; Benter, I.F. Effect of inhibition of the ubiquitin-proteasome-system and IκB kinase on airway inflammation and hyperresponsiveness in a murine model of asthma. Int. J. Immunopathol. Pharmacol. 2011, 24, 33–42. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.-R.; Ko, S.-G.; Park, H.-J.; Moon, P.-D. Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells. Antioxidants 2023, 12, 417. https://doi.org/10.3390/antiox12020417
Han N-R, Ko S-G, Park H-J, Moon P-D. Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells. Antioxidants. 2023; 12(2):417. https://doi.org/10.3390/antiox12020417
Chicago/Turabian StyleHan, Na-Ra, Seong-Gyu Ko, Hi-Joon Park, and Phil-Dong Moon. 2023. "Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells" Antioxidants 12, no. 2: 417. https://doi.org/10.3390/antiox12020417
APA StyleHan, N. -R., Ko, S. -G., Park, H. -J., & Moon, P. -D. (2023). Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells. Antioxidants, 12(2), 417. https://doi.org/10.3390/antiox12020417