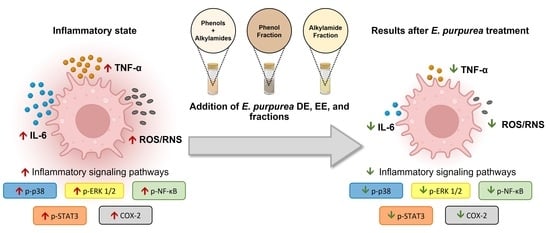
Echinacea purpurea Fractions Represent Promising Plant-Based Anti-Inflammatory Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Bioactive Compounds Extraction
2.2.1. Fractionation of Extracts
2.2.2. Characterization of Fractions Composition by LC-HRMS Analysis
2.3. Preparation of E. purpurea Extracts and Fractions Solutions
2.4. Human Monocytes
2.4.1. Ethics Statement
2.4.2. Monocyte Isolation and Differentiation
2.4.3. Evaluation of Anti-Inflammatory Activity
2.4.4. Metabolic Activity and DNA Quantification
2.4.5. Cytokine Measurement
2.4.6. Cellular ROS/RNS/O2•− Detection Assay
2.4.7. Western Blot Analysis
2.5. Statistical Analysis
3. Results
3.1. Fractionation of the E. purpurea Extracts
3.2. Chemical Composition of the E. purpurea Fractions
3.3. Cytotoxicity of E. purpurea Extracts and Fractions
3.4. Anti-Inflammatory Activity of E. purpurea Extracts and Fractions
3.4.1. Cytokine Production
3.4.2. ROS/RNS/O2•− Generation
3.4.3. Therapeutic Targets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Conner, E.M.; Grisham, M.B. Inflammation, free radicals, and antioxidants. Nutrition 1996, 12, 274–277. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, J. Pharmacological basis for the therapy of pain and inflammation with nonsteroidal anti-inflammatory drugs. Arthritis Res. 2000, 2, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles prize lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Misery, L.; Naeyaert, S.; Taylor, P.C. Biological therapies in immune-mediated inflammatory diseases: Can biosimilars reduce access inequities? Front. Pharmacol. 2019, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Chamoun-Emanuelli, A.M.; Bryan, L.K.; Cohen, N.D.; Tetrault, T.L.; Szule, J.A.; Barhoumi, R.; Whitfield-Cargile, C.M. NSAIDs disrupt intestinal homeostasis by suppressing macroautophagy in intestinal epithelial cells. Sci. Rep. 2019, 9, 14534. [Google Scholar] [CrossRef]
- Shivaji, U.N.; Sharratt, C.L.; Thomas, T.; Smith, S.C.L.; Iacucci, M.; Moran, G.W.; Ghosh, S.; Bhala, N. Review article: Managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 49, 664–680. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- WHO. WHO Monographs on Selected Medicinal Plants-Volume 1; World Health Organization: Geneva, Switzerland, 1999; ISBN 9241545178 (v.1). [Google Scholar]
- Miller, S.C. Echinacea: A miracle herb against aging and cancer? Evidence in vivo in mice. Evid. Based. Complement. Alternat. Med. 2005, 2, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [PubMed]
- Šutovská, M.; Capek, P.; Kazimierová, I.; Pappová, L.; Jošková, M.; Matulová, M.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Echinacea complex–chemical view and anti-asthmatic profile. J. Ethnopharmacol. 2015, 175, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, N.; Zhou, X.; Zhang, M.; Liu, Z.; Liu, X. Cichoric acid regulates the hepatic glucose homeostasis via AMPK pathway and activates the antioxidant response in high glucose-induced hepatocyte injury. RSC Adv. 2017, 7, 1363–1375. [Google Scholar] [CrossRef]
- Dong, G.-C.; Chuang, P.-H.; Forrest, M.D.; Lin, Y.-C.; Chen, H.M. Immuno-suppressive effect of blocking the CD28 signaling pathway in T-cells by an active component of Echinacea found by a novel pharmaceutical screening method. J. Med. Chem. 2006, 49, 1845–1854. [Google Scholar] [CrossRef]
- Gulledge, T.V.; Collette, N.M.; Mackey, E.; Johnstone, S.E.; Moazami, Y.; Todd, D.A.; Moeser, A.J.; Pierce, J.G.; Cech, N.B.; Laster, S.M. Mast cell degranulation and calcium influx are inhibited by an Echinacea purpurea extract and the alkylamide dodeca-2E,4E-dienoic acid isobutylamide. J. Ethnopharmacol. 2018, 212, 166–174. [Google Scholar]
- Hou, C.-C.; Chen, C.-H.; Yang, N.-S.; Chen, Y.-P.; Lo, C.-P.; Wang, S.-Y.; Tien, Y.-J.; Tsai, P.-W.; Shyur, L.-F. Comparative metabolomics approach coupled with cell- and gene-based assays for species classification and anti-inflammatory bioactivity validation of Echinacea plants. J. Nutr. Biochem. 2010, 21, 1045–1059. [Google Scholar] [CrossRef]
- Sasagawa, M.; Cech, N.B.; Gray, D.E.; Elmer, G.W.; Wenner, C.A. Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int. Immunopharmacol. 2006, 6, 1214–1221. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, T.; Tao, T.; Yang, J.; Chang, Y.; Wang, M.; Kim, L.; Qu, L.; Cassady, J.; Scalzo, R.; et al. Macrophage activating effects of new alkamides from the roots of Echinacea species. J. Nat. Prod. 2005, 68, 773–776. [Google Scholar] [CrossRef]
- Raduner, S.; Majewska, A.; Chen, J.-Z.; Xie, X.-Q.; Hamon, J.; Faller, B.; Altmann, K.-H.; Gertsch, J. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef]
- Hou, C.-C.; Huang, C.-C.; Shyur, L.-F. Echinacea alkamides prevent lipopolysaccharide/D-galactosamine-induced acute hepatic injury through JNK pathway-mediated HO-1 expression. J. Agric. Food Chem. 2011, 59, 11966–11974. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, W.; Wang, S.; Li, B.; Li, G.; Shi, Q. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-κB signal pathway. Int. Immunopharmacol. 2020, 88, 106974. [Google Scholar] [CrossRef] [PubMed]
- Fast, D.J.; Balles, J.A.; Scholten, J.D.; Mulder, T.; Rana, J. Echinacea purpurea root extract inhibits TNF release in response to Pam3Csk4 in a phosphatidylinositol-3-kinase dependent manner. Cell. Immunol. 2015, 297, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhu, H.; Xu, W.; Liu, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Echinacea purpurea polysaccharide prepared by fractional precipitation prevents alcoholic liver injury in mice by protecting the intestinal barrier and regulating liver-related pathways. Int. J. Biol. Macromol. 2021, 187, 143–156. [Google Scholar] [CrossRef]
- Li, Q.; Yang, F.; Hou, R.; Huang, T.; Hao, Z. Post-screening characterization of an acidic polysaccharide from Echinacea purpurea with potent anti-inflammatory properties in vivo. Food Funct. 2020, 11, 7576–7583. [Google Scholar] [CrossRef]
- Hou, R.; Xu, T.; Li, Q.; Yang, F.; Wang, C.; Huang, T.; Hao, Z. Polysaccharide from Echinacea purpurea reduce the oxidant stress in vitro and in vivo. Int. J. Biol. Macromol. 2020, 149, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, X.; Niu, Y.; Diao, Z.; Ren, B.; Li, X.; Liu, Z.; Liu, X. Cichoric acid improved hyperglycaemia and restored muscle injury via activating antioxidant response in MLD-STZ-induced diabetic mice. Food Chem. Toxicol. 2017, 107, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.-C.; Chuang, P.-H.; Chang, K.; Jan, P.; Hwang, P.-I.; Wu, H.-B.; Yi, M.; Zhou, H.-X.; Chen, H.M. Blocking effect of an immuno-suppressive agent, cynarin, on CD28 of T-cell receptor. Pharm. Res. 2009, 26, 375–381. [Google Scholar] [CrossRef]
- Cheng, Z.-Y.; Sun, X.; Liu, P.; Lin, B.; Li, L.-Z.; Yao, G.-D.; Huang, X.-X.; Song, S.-J. Sesquiterpenes from Echinacea purpurea and their anti-inflammatory activities. Phytochemistry 2020, 179, 112503. [Google Scholar] [CrossRef]
- Zhai, Z.; Solco, A.; Wu, L.; Wurtele, E.S.; Kohut, M.L.; Murphy, P.A.; Cunnick, J.E. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. J. Ethnopharmacol. 2009, 122, 76–85. [Google Scholar] [CrossRef]
- Chicca, A.; Raduner, S.; Pellati, F.; Strompen, T.; Altmann, K.-H.; Schoop, R.; Gertsch, J. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int. Immunopharmacol. 2009, 9, 850–858. [Google Scholar] [CrossRef]
- Vieira, S.F.; Gonçalves, V.M.F.; Llaguno, C.P.; Macías, F.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. On the Bioactivity of Echinacea purpurea Extracts to Modulate the Production of Inflammatory Mediators. Int. J. Mol. Sci. 2022, 23, 13616. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.F.; Ferreira, H.; Neves, N.M. Antioxidant and anti-Inflammatory activities of cytocompatible Salvia officinalis extracts: A comparison between traditional and soxhlet extraction. Antioxidants 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Epifano, F.; Contaldo, N.; Orlandini, G.; Cavicchi, L.; Genovese, S.; Bertelli, D.; Benvenuti, S.; Curini, M.; Bertaccini, A.; et al. Chromatographic methods for metabolite profiling of virus- and phytoplasma-infected plants of Echinacea purpurea. J. Agric. Food Chem. 2011, 59, 10425–10434. [Google Scholar] [CrossRef] [PubMed]
- Cech, N.B.; Eleazer, M.S.; Shoffner, L.T.; Crosswhite, M.R.; Davis, A.C.; Mortenson, A.M. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts. J. Chromatogr. A 2006, 1103, 219–228. [Google Scholar] [CrossRef]
- Spelman, K.; Wetschler, M.H.; Cech, N.B. Comparison of alkylamide yield in ethanolic extracts prepared from fresh versus dry Echinacea purpurea utilizing HPLC–ESI-MS. J. Pharm. Biomed. Anal. 2009, 49, 1141–1149. [Google Scholar] [CrossRef]
- Thomsen, M.O.; Fretté, X.C.; Christensen, K.B.; Christensen, L.P.; Grevsen, K. Seasonal variations in the concentrations of lipophilic compounds and phenolic acids in the roots of Echinacea purpurea and Echinacea pallida. J. Agric. Food Chem. 2012, 60, 12131–12141. [Google Scholar] [CrossRef]
- He, X.; Lin, L.; Bernart, M.W.; Lian, L. Analysis of alkamides in roots and achenes of Echinacea purpurea by liquid chromatography–electrospray mass spectrometry. J. Chromatogr. A 1998, 815, 205–211. [Google Scholar] [CrossRef]
- Mudge, E.; Lopes-Lutz, D.; Brown, P.; Schieber, A. Analysis of alkylamides in Echinacea plant materials and dietary supplements by ultrafast liquid chromatography with diode array and mass spectrometric detection. J. Agric. Food Chem. 2011, 59, 8086–8094. [Google Scholar] [CrossRef]
- Bauer, R.; Remiger, P. TLC and HPLC analysis of alkamides in Echinacea drugs. Planta Med. 1989, 55, 367–371. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Duarte-Oliveira, C.; Campos, C.F.; Aimanianda, V.; ter Horst, R.; Leite, L.; Mercier, T.; Pereira, P.; Fernández-García, M.; Antunes, D.; et al. Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity. Nat. Commun. 2020, 11, 2282. [Google Scholar] [CrossRef]
- Faustman, D.; Davis, M. TNF receptor 2 and disease: Autoimmunity and regenerative medicine. Front. Immunol. 2013, 4, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-L.; Chiou, S.-Y.; Chan, K.-C.; Sung, J.-M.; Lin, S.-D. Caffeic acid derivatives, total phenols, antioxidant and antimutagenic activities of Echinacea purpurea flower extracts. LWT-Food Sci. Technol. 2012, 46, 169–176. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.J.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.-R.; Chae, H.-J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Burton, G.W.; Foster, D.O.; Perly, B.; Slater, T.F.; Smith, I.C.P.; Ingold, K.U.; Willson, R.L.; Scott, G.; Norman, R.O.C.; Hill, H.A.O.; et al. Biological antioxidants. Philos. Trans. R. Soc. London. B Biol. Sci. 1985, 311, 565–578. [Google Scholar] [CrossRef]
- Porasuphatana, S.; Tsai, P.; Rosen, G.M. The generation of free radicals by nitric oxide synthase. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 281–289. [Google Scholar] [CrossRef]
- Matthias, A.; Banbury, L.; Stevenson, L.M.; Bone, K.M.; Leach, D.N.; Lehmann, R.P. Alkylamides from echinacea modulate induced immune responses in macrophages. Immunol. Investig. 2007, 36, 117–130. [Google Scholar] [CrossRef]
- Gertsch, J.; Schoop, R.; Kuenzle, U.; Suter, A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004, 577, 563–569. [Google Scholar] [CrossRef]
- Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.N.; Germoush, M.O.; Abd El-Twab, S.M.; Mahmoud, A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signaling. Environ. Sci. Pollut. Res. 2020, 27, 20725–20735. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef] [PubMed]







| Time (Min) | Water with 0.1% Formic Acid (%) | ACN (%) |
|---|---|---|
| 0 | 50 | 50 |
| 7 | 5 | 95 |
| 20 | 5 | 95 |
| 21 | 50 | 50 |
| 25 | 50 | 50 |
| Compounds | DE | EE | DE-R | DE-F | EE-F | ||||
|---|---|---|---|---|---|---|---|---|---|
| R | F | F | F2 | F2 i | F2 ii | F2 iii | F1 | F2 | |
| Malic Acid | + | - | + | - | - | - | - | - | - |
| Vanillic acid | - | - | + | - | - | - | - | - | - |
| Protocatechuic acid | - | - | + | - | - | - | - | + | - |
| Caftaric acid a | - | - | + | - | - | - | - | - | - |
| Chlorogenic acid a | - | - | + | - | - | - | - | + | - |
| Quinic acid | - | - | - | - | - | - | - | - | - |
| Vanillin | - | - | - | - | - | - | - | - | - |
| Caffeic acid a | + | + | + | - | - | - | - | + | - |
| Benzoic acid | + | + | + | - | - | - | - | - | - |
| Cynarin a | - | - | - | - | - | - | - | - | - |
| Echinacoside a | - | - | - | - | - | - | - | - | - |
| p-coumaric acid | + | - | + | - | - | - | - | - | - |
| Chicoric acid a | - | + | + | - | - | - | - | + | - |
| Rutin | - | - | + | - | - | - | - | + | - |
| Quercetin | - | - | + | - | - | - | - | - | - |
| Dodeca-2E,4Z,10E-triene-8-ynoic acid isobutylamide | + | + | + | + | + | - | - | - | + |
| Dodeca-2E,4Z,10Z-triene-8-ynoic acid isobutylamide | + | + | + | + | + | - | - | - | + |
| Dodeca-2,4,10-triene-8-ynoic acid isobutylamide (isomer 1) | + | + | - | - | - | + | - | - | - |
| Dodeca-2E,4E,10Z-triene-8-ynoic acid isobutylamide | + | + | + | + | - | - | - | - | + |
| Dodeca-2Z,4E,10Z-triene-8-ynoic acid isobutylamide | + | - | - | + | - | - | - | - | - |
| Dodeca-2E,4E,10E-triene-8-ynoic acid isobutylamide | + | + | + | + | + | - | - | - | + |
| Undeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | + | + | + | + | + | - | - | - | + |
| Undeca-2E/Z-ene-8,10-diynoic acid isobutylamidea | - | + | + | - | + | - | - | - | + |
| Undeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | + | - | - | + | - | - | - | - | - |
| Undeca-2E/Z,4Z/E-diene-8,10-diynoic acid 2-methylbutylamide | - | - | - | - | - | - | - | - | - |
| Pentadeca-2E,9Z-diene-12,14-diynoic acid 2-hydroxyisobutylamide | - | + | + | - | - | - | - | - | + |
| Dodeca-2E,4Z-diene-8,10-diynoic acid isobutylamide | + | + | + | + | + | - | - | - | + |
| Undeca-2E,4E-diene-8,10-diynoic acid isobutylamide | + | - | - | - | - | - | - | - | - |
| Dodeca-2Z,4E-diene-8,10-diynoic acid isobutylamide | - | - | - | - | - | - | - | - | - |
| Dodeca-2E-ene-8,10-diynoic acid isobutylamide a | + | + | + | + | + | - | - | - | + |
| Trideca-2E,7Z-diene-10,12-diynoic acid isobutylamide | + | + | + | + | + | - | - | - | + |
| Dodeca-2,4-diene-8,10-diynoic acid 2-methylbutylamide | + | + | + | + | + | - | - | - | + |
| Dodeca-2Z,4Z,10Z-triene-8-ynoic acid isobutylamide | + | - | - | + | - | - | - | - | - |
| Trideca-2E,7Z-diene-10,12-diynoic acid 2-methylbutylamide | + | + | + | + | - | + | - | - | + |
| Dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamide a | + | + | + | + | - | + | - | - | + |
| Dodeca-2E,4Z,10E-triene-8-ynoic acid 2-methylbutylamide OR Dodeca-2E-ene-8,10-diynoic acid 2-methylbutylamide | + | + | + | + | + | - | - | - | + |
| Dodeca-2E,4E,8Z-trienoic acid isobutylamide (isomer 1) | - | + | + | - | - | - | + | - | - |
| Dodeca-2E,4E-dienoic acid isobutylamide (isomer 1) | - | - | - | - | - | - | - | - | - |
| Pentadeca-2E,9Z-diene-12,14-diynoic acid isobutylamide | + | + | + | + | - | + | - | - | + |
| Dodeca-2E,4E,8Z-trienoic acid isobutylamide | + | + | + | + | - | - | + | - | + |
| Trideca-2Z,7Z-diene-10,12-diynoic acid 2-methylbutylamide | + | - | - | - | - | - | - | - | - |
| Dodeca-2E,4E,8Z,10E/Z-tetraenoic acid 2-methylbutylamide | + | + | + | + | - | - | + | - | + |
| Hexadeca-2E,9Z-diene-12,14-diynoic acid isobutylamide | + | - | - | - | - | - | - | - | - |
| Dodeca-2E,4E,8Z-trienoic acid isobutylamide (isomer 2) | + | - | - | - | - | - | - | - | - |
| Dodeca-2E,4E-dienoic acid isobutylamide a | + | + | + | + | - | - | + | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, S.F.; Gonçalves, S.M.; Gonçalves, V.M.F.; Llaguno, C.P.; Macías, F.; Tiritan, M.E.; Cunha, C.; Carvalho, A.; Reis, R.L.; Ferreira, H.; et al. Echinacea purpurea Fractions Represent Promising Plant-Based Anti-Inflammatory Formulations. Antioxidants 2023, 12, 425. https://doi.org/10.3390/antiox12020425
Vieira SF, Gonçalves SM, Gonçalves VMF, Llaguno CP, Macías F, Tiritan ME, Cunha C, Carvalho A, Reis RL, Ferreira H, et al. Echinacea purpurea Fractions Represent Promising Plant-Based Anti-Inflammatory Formulations. Antioxidants. 2023; 12(2):425. https://doi.org/10.3390/antiox12020425
Chicago/Turabian StyleVieira, Sara F., Samuel M. Gonçalves, Virgínia M. F. Gonçalves, Carmen P. Llaguno, Felipe Macías, Maria Elizabeth Tiritan, Cristina Cunha, Agostinho Carvalho, Rui L. Reis, Helena Ferreira, and et al. 2023. "Echinacea purpurea Fractions Represent Promising Plant-Based Anti-Inflammatory Formulations" Antioxidants 12, no. 2: 425. https://doi.org/10.3390/antiox12020425
APA StyleVieira, S. F., Gonçalves, S. M., Gonçalves, V. M. F., Llaguno, C. P., Macías, F., Tiritan, M. E., Cunha, C., Carvalho, A., Reis, R. L., Ferreira, H., & Neves, N. M. (2023). Echinacea purpurea Fractions Represent Promising Plant-Based Anti-Inflammatory Formulations. Antioxidants, 12(2), 425. https://doi.org/10.3390/antiox12020425