Redox Signaling in Plant Heat Stress Response
Abstract
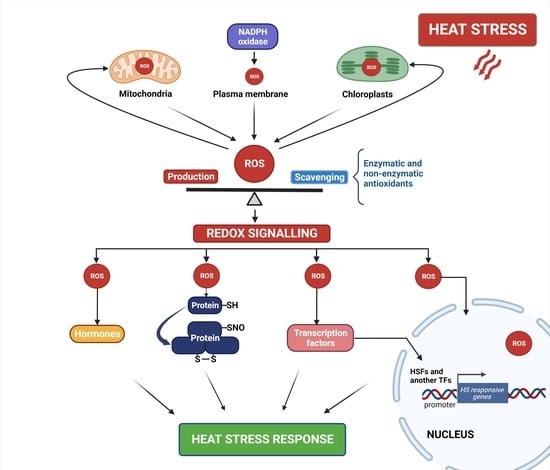
:1. Introduction
2. Production of Reactive Oxygen Species in Heat Stress Conditions
3. Role of Antioxidants in Heat Stress Response
4. Reactive Oxygen Species as Signaling Molecules in the Heat Stress Response
5. Heat Stress-Dependent Crosstalk between Signaling Networks of Reactive Oxygen Species and Hormones
6. Heat Stress-Dependent Oxidation of Cellular Environment
7. Redox-Dependent Changes of Proteins Involved in the Heat Stress Response
8. Redox-Dependent Changes of Proteins Impacting Gene Expression during Heat Stress Response
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Larminat, P. Earth Climate Identification vs. Anthropic Global Warming Attribution. Annu. Rev. Control 2016, 42, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, In Press. [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cook, B.I.; Allen, J.M.; Crimmins, T.M.; Betancourt, J.L.; Travers, S.E.; Pau, S.; Regetz, J.; Davies, T.J.; Kraft, N.J.B.; et al. Warming Experiments Underpredict Plant Phenological Responses to Climate Change. Nature 2012, 485, 494–497. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding Rnas, and Epigenetics. Int. J. Mol. Sci. 2021, 22, 117. [Google Scholar] [CrossRef]
- Wang, L.; Ma, K.B.; Lu, Z.G.; Ren, S.X.; Jiang, H.R.; Cui, J.W.; Chen, G.; Teng, N.J.; Lam, H.M.; Jin, B. Differential Physiological, Transcriptomic and Metabolomic Responses of Arabidopsis Leaves under Prolonged Warming and Heat Shock. BMC Plant Biol. 2020, 20, 86. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Murty, M.V.R.; Quick, W.P. Rice Responses to Rising Temperatures—Challenges, Perspectives and Future Directions. Plant Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef]
- Ullah, A.; Nadeem, F.; Nawaz, A.; Siddique, K.H.M.; Farooq, M. Heat Stress Effects on the Reproductive Physiology and Yield of Wheat. J. Agron. Crop Sci. 2022, 208, 1–17. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yu, J.Q. Plant Hormones under Challenging Environmental Factors; Springer Dordrecht: Berlin, Germany, 2016; pp. 1–269. [Google Scholar] [CrossRef]
- Peck, S.; Mittler, R. Plant Signaling in Biotic and Abiotic Stress. J. Exp. Bot. 2020, 71, 1649–1651. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Dish, P.; Patel, M. Effect of Abiotic Stress on Crops. Sustain. Crop Prod. 2020, 3. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z. Reactive Oxygen Species Signaling and Stomatal Movement in Plant Responses to Drought Stress and Pathogen Attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat Stress in Cultivated Plants: Nature, Impact, Mechanisms, and Mitigation Strategies—A Review. Plant Biosyst. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat Stress Decreases Levels of Nutrient-Uptake and -Assimilation Proteins in Tomato Roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef]
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of Wild and Cultivated Plants under Global Warming Conditions. Curr. Biol. 2019, 29, R1326–R1338. [Google Scholar] [CrossRef]
- Sharkey, T.D. Effects of Moderate Heat Stress on Photosynthesis: Importance of Thylakoid Reactions, Rubisco Deactivation, Reactive Oxygen Species, and Thermotolerance Provided by Isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat Stress: An Overview of Molecular Responses in Photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of Photosystem II under Environmental Stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Murata, N. How Do Environmental Stresses Accelerate Photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ryota, A.E.; Ae, A.; Yoshioka, M.; Mahbuba, A.E.; Ae, K.; Komayama, K.; Daichi, A.E.; Ae, T.; Yamashita, A.; et al. Quality Control of Photosystem II: Impact of Light and Heat Stresses. Photosynth. Res. 2008, 98, 589–608. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Zhu, H.; Huang, M.; Zhu, Q.; Tang, D.; Han, X.; Li, J.; Sun, J.; Fu, J. Ascorbic Acid Alleviates Damage from Heat Stress in the Photosystem II of Tall Fescue in Both the Photochemical and Thermal Phases. Front. Plant Sci. 2017, 8, 1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamori, W.; Takahashi, S.; Makino, A.; Price, G.D.; Badger, M.R.; von Caemmerer, S. The Roles of ATP Synthase and the Cytochrome b6/f Complexes in Limiting Chloroplast Electron Transport and Determining Photosynthetic Capacity. Plant Physiol. 2011, 155, 956–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, T.D.; Zhang, R. High Temperature Effects on Electron and Proton Circuits of Photosynthesis. J. Integr. Plant Biol. 2010, 52, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Jajoo, A.; Mehta, P.; Bharti, S. Analysis of Elevated Temperature-induced Inhibition of Photosystem II Using Chlorophyll a Fluorescence Induction Kinetics in Wheat Leaves (Triticum Aestivum). Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- Khanna-Chopra, R. Leaf Senescence and Abiotic Stresses Share Reactive Oxygen Species-Mediated Chloroplast Degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef]
- Rossi, S.; Burgess, P.; Jespersen, D.; Huang, B. Heat-Induced Leaf Senescence Associated with Chlorophyll Metabolism in Bentgrass Lines Differing in Heat Tolerance. Crop Sci. 2017, 57, S-169–S-178. [Google Scholar] [CrossRef]
- Liang, M.H.; Jiang, J.G.; Wang, L.; Zhu, J. Transcriptomic Insights into the Heat Stress Response of Dunaliella Bardawil. Enzyme Microb. Technol. 2020, 132, 109436. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Prasch, C.M.; Sonnewald, U. Signaling Events in Plants: Stress Factors in Combination Change the Picture. Environ. Exp. Bot. 2015, 114, 4–14. [Google Scholar] [CrossRef]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular Mechanisms of Plant Tolerance to Heat Stress: Current Landscape and Future Perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigot, S.; Buges, J.; Gilly, L.; Jacques, C.; Le Boulch, P.; Berger, M.; Delcros, P.; Domergue, J.B.; Koehl, A.; Ley-Ngardigal, B.; et al. Pivotal Roles of Environmental Sensing and Signaling Mechanisms in Plant Responses to Climate Change. Glob. Chang. Biol. 2018, 24, 5573–5589. [Google Scholar] [CrossRef] [PubMed]
- Raza, A. Metabolomics: A Systems Biology Approach for Enhancing Heat Stress Tolerance in Plants. Plant Cell Rep. 2020, 41, 741–763. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Dickinson, P.J.; Kumar, M.; Martinho, C.; Yoo, S.J.; Lan, H.; Artavanis, G.; Charoensawan, V.; Schöttler, M.A.; Bock, R.; Jaeger, K.E.; et al. Chloroplast Signaling Gates Thermotolerance in Arabidopsis. Cell Rep. 2018, 22, 1657–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fey, V.; Wagner, R.; Bräutigam, K.; Wirtz, M.; Hell, R.; Dietzmann, A.; Leister, D.; Oelmüller, R.; Pfannschmidt, T. Retrograde Plastid Redox Signals in the Expression of Nuclear Genes for Chloroplast Proteins of Arabidopsis Thaliana. J. Biol. Chem. 2005, 280, 5318–5328. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.-Z.; Guo, F.-Q. Chloroplast Retrograde Regulation of Heat Stress Responses in Plants. Front. Plant Sci. 2016, 7, 398. [Google Scholar] [CrossRef] [Green Version]
- Haslbeck, M.; Vierling, E. A First Line of Stress Defense: Small Heat Shock Proteins and Their Function in Protein Homeostasis. J. Mol. Biol. 2015, 427, 1537–1548. [Google Scholar] [CrossRef] [Green Version]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The Plant Heat Stress Transcription Factor (Hsf) Family: Structure, Function and Evolution. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Suzuki, N. Fine Tuning of ROS, Redox and Energy Regulatory Systems Associated with the Functions of Chloroplasts and Mitochondria in Plants under Heat Stress. Int. J. Mol. Sci. 2023, 24, 1356. [Google Scholar] [CrossRef]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-Level Interactions between Heat Shock Factors, Heat Shock Proteins, and the Redox System Regulate Acclimation to Heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The Roles of Reactive Oxygen Metabolism in Drought: Not so Cut and Dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnocka, W.; Karpiński, S. Friend or Foe? Reactive Oxygen Species Production, Scavenging and Signaling in Plant Response to Environmental Stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, H.; Munné-Bosch, S. Production and Scavenging of Reactive Oxygen Species and Redox Signaling during Leaf and Flower Senescence: Similar but Different. Plant Physiol. 2016, 171, 1560–1568. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Doğru, A. Effects of Heat Stress on Photosystem II Activity and Antioxidant Enzymes in Two Maize Cultivars. Planta 2021, 253, 85. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet Oxygen Production in Photosystem II and Related Protection Mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Nijo, N.; Pospíšil, P.; Morita, N.; Takenaka, D.; Aminaka, R.; Yamamoto, Y.; Yamamoto, Y. Quality Control of Photosystem II reactive oxygen species are responsible for the damage to photosystem ii under moderate heat stress. J. Biol. Chem. 2008, 283, 28380–28391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.; Ferretti, U.; Sedlaová, M.; Pospíšil, P. Singlet Oxygen Production in Chlamydomonas Reinhardtii under Heat Stress. Sci. Rep. 2016, 6, 20094. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R. The Oxygen-Evolving Complex: A Super Catalyst for Life on Earth, in Response to Abiotic Stresses. Plant Signal. Behav. 2020, 15, 1824721. [Google Scholar] [CrossRef]
- Yadav, D.K.; Pospíšil, P. Role of Chloride Ion in Hydroxyl Radical Production in Photosystem II under Heat Stress: Electron Paramagnetic Resonance Spin-Trapping Study. J. Bioenerg. Biomembr. 2012, 44, 365–372. [Google Scholar] [CrossRef]
- Vacca, R.A.; de Pinto, M.C.; Valenti, D.; Passarella, S.; Marra, E.; De Gara, L. Production of Reactive Oxygen Species, Alteration of Cytosolic Ascorbate Peroxidase, and Impairment of Mitochondrial Metabolism Are Early Events in Heat Shock-Induced Programmed Cell Death in Tobacco Bright-Yellow 2 Cells. Plant Physiol. 2004, 134, 1100. [Google Scholar] [CrossRef] [Green Version]
- Valenti, D.; Vacca, R.A.; de Pinto, M.C.; De Gara, L.; Marra, E.; Passarella, S. In the Early Phase of Programmed Cell Death in Tobacco Bright Yellow 2 Cells the Mitochondrial Adenine Nucleotide Translocator, Adenylate Kinase and Nucleoside Diphosphate Kinase Are Impaired in a Reactive Oxygen Species-Dependent Manner. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Cvetkovska, M.; Alber, N.A.; Vanlerberghe, G.C. The Signaling Role of a Mitochondrial Superoxide Burst during Stress. Plant Signal. Behav. 2013, 8, 1121–1136. [Google Scholar] [CrossRef] [Green Version]
- Rikhvanov, E.G.; Fedoseeva, I.V.; Pyatrikas, D.V.; Borovskii, G.B.; Voinikov, V.K. Role of Mitochondria in the Operation of Calcium Signaling System in Heat-Stressed Plants. Russ. J. Plant Physiol. 2014, 61, 141–153. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Fan, Y.; Posch, B.C.; Garcia, A.; Coast, O.; Atkin, O.K. Responses of Leaf Respiration to Heatwaves. Plant Cell Environ. 2021, 44, 2090–2101. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Wei, S.S.; Niu, W.T.; Zhai, X.T.; Liang, W.Q.; Xu, M.; Fan, X.; Lv, T.T.; Xu, W.Y.; Bai, J.T.; Jia, N.; et al. Arabidopsis MtHSC70-1 Plays Important Roles in the Establishment of COX-Dependent Respiration and Redox Homeostasis. J. Exp. Bot. 2019, 70, 5575–5590. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Vanlerberghe, G.C. Alternative Oxidase Modulates Leaf Mitochondrial Concentrations of Superoxide and Nitric Oxide. New Phytol. 2012, 195, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Borovik, O.A.; Grabelnych, O.I. Mitochondrial Alternative Cyanide-Resistant Oxidase Is Involved in an Increase of Heat Stress Tolerance in Spring Wheat. J. Plant Physiol. 2018, 231, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Production of Reactive Oxygen Species by Plant. Structure 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory Burst Oxidases: The Engines of ROS Signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The Plant NADPH Oxidase RBOHD Mediates Rapid Systemic Signaling in Response to Diverse Stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [Green Version]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat Stress Phenotypes of Arabidopsis Mutants Implicate Multiple Signaling Pathways in the Acquisition of Thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [Green Version]
- Suriyasak, C.; Harano, K.; Tanamachi, K.; Matsuo, K.; Tamada, A.; Iwaya-inoue, M.; Ishibashi, Y. Reactive Oxygen Species Induced by Heat Stress during Grain Fi Lling of Rice (Oryza Sativa L.) Are Involved in Occurrence of Grain Chalkiness. J. Plant Physiol. 2017, 216, 52–57. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, F.; Cen, B.; Wen, J.; Zhou, Y.; Wu, Z. Respiratory Burst Oxidase Homologue-Dependent H2O2 and Chloroplast H2O2 Are Essential for the Maintenance of Acquired Thermotolerance during Recovery after Acclimation. Plant Cell Environ. 2018, 41, 2373–2389. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Signaling in Plants. Antioxidants Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Zhang, W.; Ma, S.; Jiang, D.; Han, X. Heat Priming during Early Reproductive Stages Enhances Thermo-Tolerance to Post-Anthesis Heat Stress via Improving Photosynthesis and Plant Productivity in Winter Wheat (Triticum Aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple Heat Priming Enhances Thermo-Tolerance to a Later High Temperature Stress via Improving Subcellular Antioxidant Activities in Wheat Seedlings. Plant Physiol. Biochem. PPB 2014, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Boyle, D.L.; Welti, R.; Jagadish, S.V.K.; Prasad, P.V.V. Decreased Photosynthetic Rate under High Temperature in Wheat Is Due to Lipid Desaturation, Oxidation, Acylation, and Damage of Organelles. BMC Plant Biol. 2018, 18, 55. [Google Scholar] [CrossRef] [Green Version]
- Huo, L.; Sun, X.; Guo, Z.; Jia, X.; Che, R.; Sun, Y.; Zhu, Y.; Wang, P.; Gong, X.; Ma, F. MdATG18a Overexpression Improves Basal Thermotolerance in Transgenic Apple by Decreasing Damage to Chloroplasts. Hortic. Res. 2020, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.L.; Chen, Q.Q. Exogenous Sucrose Protects Potato Seedlings Against Heat Stress by Enhancing the Antioxidant Defense System. J. Soil Sci. Plant Nutr. 2021, 21, 1511–1519. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Imran, M.; Asaf, S.; Kim, Y.H.; Bilal, S.; Numan, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-Induced Thermotolerance in Solanum Lycopersicum L. via Activation of Antioxidant System, Heat Shock Proteins, and Endogenous Phytohormones. BMC Plant Biol. 2020, 20, 248. [Google Scholar] [CrossRef]
- Christou, A.; Filippou, P.; Manganaris, G.A.; Fotopoulos, V. Sodium Hydrosulfide Induces Systemic Thermotolerance to Strawberry Plants through Transcriptional Regulation of Heat Shock Proteins and Aquaporin. BMC Plant Biol. 2014, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Li, J.; Zhu, C.; Jing, B.; Shi, K.; Yu, J.; Hu, Z. Exogenous Rosmarinic Acid Application Enhances Thermotolerance in Tomatoes. Plants 2022, 11, 1172. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, J.; Grewal, S.K.; Singh, I. Effect of Heat Stress on Antioxidative Defense System and Its Amelioration by Heat Acclimation and Salicylic Acid Pre-Treatments in Three Pigeonpea Genotypes. Indian J. Agric. Biochem. 2019, 32, 106–110. [Google Scholar] [CrossRef]
- Hameed, A.; Goher, M.; Iqbal, N. Heat Stress-Induced Cell Death, Changes in Antioxidants, Lipid Peroxidation, and Protease Activity in Wheat Leaves. J. Plant Growth Regul. 2012, 31, 283–291. [Google Scholar] [CrossRef]
- de Pinto, M.C.; Locato, V.; Paradiso, A.; De Gara, L. Role of Redox Homeostasis in Thermo-Tolerance under a Climate Change Scenario. Ann. Bot. 2015, 116, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.K.; Khan, A.; Maheshwari, A.; Narayan, S.; Chhapola, O.P.; Arora, A.; Singh, G. Effect of Post Anthesis High Temperature Stress on Growth, Physiology and Antioxidative Defense Mechanisms in Contrasting Wheat Genotypes. Indian J. Plant Physiol. 2015, 20, 103–110. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, S.; Naz, N.; Shah, M. Effect of Heat Stress on Growth, Physiological and Biochemical Activities of Wheat (Triticum Aestivum L.). Int. J. Biosci. 2017, 11, 173–183. [Google Scholar] [CrossRef]
- Rani, B.; Kumari, N.; Pooja, D.; Jain, V.; Dhawan, K.; Monika, R.A.; Kumar, A.; Sheoran, P. Antioxidative System as Influenced by High Temperature Stress in Brassica Juncea (L) Czern & Coss. Curr. Trends Biotechnol. Pharm. 2016, 10, 118–125. [Google Scholar]
- Yang, Z.; Mhamdi, A.; Noctor, G. Analysis of Catalase Mutants Underscores the Essential Role of CATALASE2 for Plant Growth and Day Length-Dependent Oxidative Signalling. Plant Cell Environ. 2019, 42, 688–700. [Google Scholar] [CrossRef]
- Ono, M.; Isono, K.; Sakata, Y.; Taji, T. Biochemical and Biophysical Research Communications CATALASE2 Plays a Crucial Role in Long-Term Heat Tolerance of Arabidopsis Thaliana. Biochem. Biophys. Res. Commun. 2021, 534, 747–751. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Modulation of Antioxidant Machinery and the Methylglyoxal Detoxification System in Selenium-Supplemented Brassica Napus Seedlings Confers Tolerance to High Temperature Stress. Biol. Trace Elem. Res. 2014, 161, 297–307. [Google Scholar] [CrossRef]
- Maruta, T.; Sawa, Y.; Shigeoka, S.; Ishikawa, T. Diversity and Evolution of Ascorbate Peroxidase Functions in Chloroplasts: More than Just a Classical Antioxidant Enzyme? Plant Cell Physiol. 2016, 57, 1377–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkov, R.A.; Panchuk, I.I.; Mullineaux, P.M.; Schöffl, F. Heat Stress-Induced H2O2 Is Required for Effective Expression of Heat Shock Genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef]
- Panchuk, I.I.; Volkov, R.A.; Schöffl, F. Heat Stress- and Heat Shock Transcription Factor-Dependent Expression and Activity of Ascorbate Peroxidase in Arabidopsis. Plant Physiol. 2002, 129, 838–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, F.; Ganguli, A.; Kiehlmann, E.; Englich, G.; Walch, D.; Von Koskull-Döring, P. The Heat Stress Transcription Factor HsfA2 Serves as a Regulatory Amplifier of a Subset of Genes in the Heat Stress Response in Arabidopsis. Plant Mol. Biol. 2006, 60, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.A.; Kumar, B.; Reddy, P.S.; Mishra, R.N.; Mahanty, S.; Kaul, T.; Nair, S.; Sopory, S.K.; Reddy, M.K. Molecular Cloning and Characterization of Genes Encoding Pennisetum Glaucum Ascorbate Peroxidase and Heat-Shock Factor: Interlinking Oxidative and Heat-Stress Responses. J. Plant Physiol. 2009, 166, 1646–1659. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Sejima, H.; Harper, J.; Mittler, R. Enhanced Seed Production under Prolonged Heat Stress Conditions in Arabidopsis Thaliana Plants Deficient in Cytosolic Ascorbate Peroxidase 2. J. Exp. Bot. 2013, 64, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Li, J.; Ding, S.; Cheng, F.; Li, X.; Jiang, Y.; Yu, J.; Foyer, C.H.; Shi, K. The Protein Kinase CPK28 Phosphorylates Ascorbate Peroxidase and Enhances Thermotolerance in Tomato. Plant Physiol. 2021, 186, 1302–1317. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double Mutants Deficient in Cytosolic and Thylakoid Ascorbate Peroxidase Reveal a Complex Mode of Interaction between Reactive Oxygen Species, Plant Development, and Response to Abiotic Stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate Peroxidase 1 Plays a Key Role in the Response of Arabidopsis Thaliana to Stress Combination S. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA Is Required for the Accumulation of APX1 and MBF1c during a Combination of Water Deficit and Heat Stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Prakash, P.; Bak, D.-H.; Hong, S.H.; Cho, C.; Chung, M.-S.; Kim, J.-H.; Lee, S.; Bai, H.-W.; Lee, S.Y.; et al. Regulation of Dual Activity of Ascorbate Peroxidase 1 From Arabidopsis Thaliana by Conformational Changes and Posttranslational Modifications. Front. Plant Sci. 2021, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Babbar, R.; Karpinska, B.; Grover, A.; Foyer, C.H. Heat-Induced Oxidation of the Nuclei and Cytosol. Front. Plant Sci. 2021, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; Gadaleta, C.; De Gara, L.; de Pinto, M.C. Production of Reactive Species and Modulation of Antioxidant Network in Response to Heat Shock: A Critical Balance for Cell Fate. Plant Cell Environ. 2008, 31, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; de Pinto, M.C.; De Gara, L. Different Involvement of the Mitochondrial, Plastidial and Cytosolic Ascorbate–Glutathione Redox Enzymes in Heat Shock Responses. Physiol. Plant. 2009, 135, 296–306. [Google Scholar] [CrossRef]
- de Pinto, M.C.; Locato, V.; Sgobba, A.; Romero-Puertas, M.; del, C.; Gadaleta, C.; Delledonne, M.; De Gara, L. S-Nitrosylation of Ascorbate Peroxidase Is Part of Programmed Cell Death Signaling in Tobacco Bright Yellow-2 Cells. Plant Physiol. 2013, 163, 1766–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgobba, A.; Paradiso, A.; Dipierro, S.; de Gara, L.; de Pinto, M.C. Changes in Antioxidants Are Critical in Determining Cell Responses to Short- and Long-Term Heat Stress. Physiol. Plant. 2015, 153, 68–78. [Google Scholar] [CrossRef]
- Camejo, D.; Martí, D.C.; Nicolá, E.; Alarcó, N.B.J.J.; Jimé Nez, A.; Sevilla, F. Response of Superoxide Dismutase Isoenzymes in Tomato Plants (Lycopersicon Esculentum) during Thermo-Acclimation of the Photosynthetic Apparatus. Physiol. Plant. 2007, 131, 367. [Google Scholar] [CrossRef]
- Camejo, D.; Jiménez, A.; Alarcón, J.J.; Torres, W.; Gómez, J.M.; Sevilla, F. Changes in Photosynthetic Parameters and Antioxidant Activities Following Heat-Shock Treatment in Tomato Plants. Funct. Plant Biol. 2006, 33, 177–187. [Google Scholar] [CrossRef]
- Guan, Q.; Lu, X.; Zeng, H.; Zhang, Y.; Zhu, J. Heat Stress Induction of MiR398 Triggers a Regulatory Loop That Is Critical for Thermotolerance in Arabidopsis. Plant J. 2013, 74, 840–851. [Google Scholar] [CrossRef]
- Lasorella, C.; Fortunato, S.; Dipierro, N.; Jeran, N.; Tadini, L.; Vita, F.; Pesaresi, P.; de Pinto, M.C. Chloroplast-Localized GUN1 Contributes to the Acquisition of Basal Thermotolerance in Arabidopsis Thaliana. Front. Plant Sci. 2022, 13, 1058831. [Google Scholar] [CrossRef]
- Kong, F.; Deng, Y.; Wang, G.; Wang, J.; Liang, X.; Meng, Q. LeCDJ1, a Chloroplast DnaJ Protein, Facilitates Heat Tolerance in Transgenic Tomatoes. J. Integr. Plant Biol. 2014, 56, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cai, G.; Xu, N.; Zhang, L.; Sun, X.; Guan, J.; Meng, Q. Novel DnaJ Protein Facilitates Thermotolerance of Transgenic Tomatoes. Int. J. Mol. Sci. 2019, 20, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, H.; Xie, Q.; Liu, Y.; Lv, H.; Bai, R.; Ma, R.; Li, X.; Zhang, X.; Guo, Y.D.; et al. SlSNAT Interacts with HSP40, a Molecular Chaperone, to Regulate Melatonin Biosynthesis and Promote Thermotolerance in Tomato. Plant Cell Physiol. 2020, 61, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xing, M.; Hu, T.; Ji, M.; Li, X.; Amombo, E.; Shao, A.; Xu, X.; Fu, J. Photosystem II Photochemical Adjustment of Tall Fescue against Heat Stress after Melatonin Priming. J. Plant Physiol. 2022, 275, 153758. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous Glutathione Confers High Temperature Stress Tolerance in Mung Bean (Vigna Radiata L.) by Modulating Antioxidant Defense and Methylglyoxal Detoxification System. Environ. Exp. Bot. 2014, 112, 44–54. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of Drought and Heat Stress Individually and in Combination on Physio-Biochemical Parameters, Antioxidant Responses, and Gene Expression in Solanum Lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Fujit, M. Extreme Temperature Responses, Oxidative Stress and Antioxidant Defense in Plants. In Abiotic Stress—Plant Responses and Applications in Agriculture; InTechOpen: London, UK, 2013; pp. 169–205. [Google Scholar] [CrossRef] [Green Version]
- Eyshi Rezaei, E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat Stress in Cereals: Mechanisms and Modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Banti, V.; Mafessoni, F.; Loreti, E.; Alpi, A.; Perata, P. The Heat-Inducible Transcription Factor HsfA2 Enhances Anoxia Tolerance in Arabidopsis. Plant Physiol. 2010, 152, 1471. [Google Scholar] [CrossRef] [Green Version]
- Königshofer, H.; Tromballa, H.-W.; Löppert, H.-G. Early Events in Signalling High-Temperature Stress in Tobacco BY2 Cells Involve Alterations in Membrane Fluidity and Enhanced Hydrogen Peroxide Production. Plant Cell Environ. 2008, 31, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, Y.; Jia, L.; Chu, H.; Zhou, S.; Chen, K.; Wu, D.; Zhao, L. Hydrogen Peroxide Acts Upstream of Nitric Oxide in the Heat Shock Pathway in Arabidopsis Seedlings. Plant Physiol. 2014, 164, 2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen Peroxide Sensor HPCA1 Is an LRR Receptor Kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, G.; Zhang, S.; Li, Y.; Gu, H.; Li, Y.; Zhao, Q.; Qi, Y. Chloroplast-to-Nucleus Signaling Regulates MicroRNA Biogenesis in Arabidopsis. Dev. Cell 2019, 48, 371–382.e4. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Jia, T.; Gu, T.; Su, J.; Hu, X. Progress in Research on the Mechanisms Underlying. Genes 2021, 12, 1343. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid Oxidation Products Are Stress Signals That Mediate Gene Responses to Singlet Oxygen in Plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef] [Green Version]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-Triggered Retrograde Signaling from Chloroplasts to Nucleus Plays Specific Role in Response to Stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [Green Version]
- Koussevitzky, S.; Nott, A.; Mockler, T.C.; Hong, F.; Sachetto-Martins, G.; Surpin, M.; Lim, J.; Mittler, R.; Chory, J. Signals from Chloroplasts Converge to Regulate Nuclear Gene Expression. Science 2007, 316, 715–719. [Google Scholar] [CrossRef]
- Wu, G.Z.; Chalvin, C.; Hoelscher, M.; Meyer, E.H.; Wu, X.N.; Bock, R. Control of Retrograde Signaling by Rapid Turnover of GENOMES UNCOUPLED1. Plant Physiol. 2018, 176, 2472–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesaresi, P.; Kim, C. Current Understanding of GUN1: A Key Mediator Involved in Biogenic Retrograde Signaling. Plant Cell Rep. 2019, 38, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.M.; Kozuleva, M.A.; Rudenko, N.N.; Naydov, I.A.; Klenina, I.B.; Ivanov, B.N. Photosynthetic Electron Flow to Oxygen and Diffusion of Hydrogen Peroxide through the Chloroplast Envelope via Aquaporins. Proc. Biochim. et Biophys. Acta Bioenerg. 2012, 1817, 1314–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N.; Park, E. Spatial Chloroplast-to-Nucleus Signalling Involving Plastid-Nuclear Complexes and Stromules. Philos. Trans. R Soc. Lond. B Biol. Sci. 2020, 375, 20190405. [Google Scholar] [CrossRef]
- Breeze, E.; Mullineaux, P.M. The Passage of H2O2 from Chloroplasts to Their Associated Nucleus during Retrograde Signalling: Reflections on the Role of the Nuclear Envelope. Plants 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of Reactive Oxygen Species and Hormone Signaling during Abiotic Stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between Reactive Oxygen Species and Hormones in the Control of Plant Development and Stress Tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [Green Version]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Sengupta, S.; Burks, D.; Azad, R.K.; Mittler, R. Identification and Characterization of a Core Set of ROS Wave-Associated Transcripts Involved in the Systemic Acquired Acclimation Response of Arabidopsis to Excess Light. Plant J. 2019, 98, 126–141. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic Signaling during Abiotic Stress Combination in Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. H2O2 Mediates the Crosstalk of Brassinosteroid and Abscisic Acid in Tomato Responses to Heat and Oxidative Stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional Analysis of Arabidopsis Mutants Points to Novel Roles for Glutathione in Coupling H2O2 to Activation of Salicylic Acid Accumulation and Signaling. Antioxid. Redox Signal. 2013, 18, 2106–2121. [Google Scholar] [CrossRef] [Green Version]
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.E.N.; Nakamura, Y.; Murata, Y. Guard Cell Salicylic Acid Signaling Is Integrated into Abscisic Acid Signaling via the Ca2+/CPK-Dependent Pathway. Plant Physiol. 2018, 178, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, H.J.; Jung, J.H.; Park, C.M. The Arabidopsis Thaliana RNA-Binding Protein FCA Regulates Thermotolerance by Modulating the Detoxification of Reactive Oxygen Species. New Phytol. 2015, 205, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Obata, T.; Feil, R.; Lunn, J.E.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Fernie, A.R. The Role of Abscisic Acid Signaling in Maintaining the Metabolic Balance Required for Arabidopsis Growth under Nonstress Conditions. Plant Cell. 2019, 31, 84–105. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; He, R.J.; Xie, Q.L.; Zhao, X.h.; Deng, X.m.; He, J.b.; Song, L.; He, J.; Marchant, A.; Chen, X.Y.; et al. ETHYLENE RESPONSE FACTOR 74 (ERF74) Plays an Essential Role in Controlling a Respiratory Burst Oxidase Homolog D (RbohD)-Dependent Mechanism in Response to Different Stresses in Arabidopsis. New Phytol. 2017, 213, 1667–1681. [Google Scholar] [CrossRef]
- Suzuki, N.; Bajad, S.; Shuman, J.; Shulaev, V.; Mittler, R. The Transcriptional Co-Activator MBF1c Is a Key Regulator of Thermotolerance in Arabidopsis Thaliana. J. Biol. Chem. 2008, 283, 9269–9275. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Signal Transduction Networks during Stress Combination. J. Exp. Bot. 2020, 71, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Letnik, I.; Hacham, Y.; Dobrev, P.; Ben-Daniel, B.H.; Vanková, R.; Amir, R.; Miller, G. ASCORBATE PEROXIDASE6 Protects Arabidopsis Desiccating and Germinating Seeds from Stress and Mediates Cross Talk between Reactive Oxygen Species, Abscisic Acid, And Auxin. Plant Physiol. 2014, 166, 370–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 Transcription Factor Regulates Heat Stress Tolerance through FERONIA Receptor-like Kinase-Mediated Reactive Oxygen Species Signaling in Tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.J.; Cheng, J.S.; Luo, H.B.; Li, S.H. Salicylic Acid Alleviates Decreases in Photosynthesis under Heat Stress and Accelerates Recovery in Grapevine Leaves. BMC Plant Biol. 2010, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, J.; Lee, K.P.; Dogra, V.; Zhang, S.; Liu, K.; Caceres-Moreno, C.; Lv, S.; Xing, W.; Kato, Y.; Sakamoto, W.; et al. Impaired Psii Proteostasis Promotes Retrograde Signaling via Salicylic Acid1. Plant Physiol. 2019, 180, 2182–2197. [Google Scholar] [CrossRef]
- Suzuki, N.; Katano, K. Coordination between ROS Regulatory Systems and Other Pathways under Heat Stress and Pathogen Attack. Front. Plant Sci. 2018, 9, 490. [Google Scholar] [CrossRef]
- Shah Jahan, M.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous Salicylic Acid Increases the Heat Tolerance in Tomato (Solanum Lycopersicum L) by Enhancing Photosynthesis Efficiency and Improving Antioxidant Defense System through Scavenging of Reactive Oxygen Species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Torun, H.; Novák, O.; Mikulík, J.; Pěnčík, A.; Strnad, M.; Ayaz, F.A. Timing-Dependent Effects of Salicylic Acid Treatment on Phytohormonal Changes, ROS Regulation, and Antioxidant Defense in Salinized Barley (Hordeum Vulgare L.). Sci. Rep. 2020, 10, 13886. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the Detoxification of HT-Induced ROS Burst in Rice Anther and Its Relation to Pollen Fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic Acid Reverses Pollen Abortion of Rice Caused by Heat Stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Valluru, R.; Reynolds, M.P.; Davies, W.J.; Sukumaran, S. Phenotypic and Genome-Wide Association Analysis of Spike Ethylene in Diverse Wheat Genotypes under Heat Stress. New Phytol. 2017, 214, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wi, S.J.; Jang, S.J.; Park, K.Y. Inhibition of Biphasic Ethylene Production Enhances Tolerance to Abiotic Stress by Reducing the Accumulation of Reactive Oxygen Species in Nicotiana Tabacum. Mol. Cells 2010, 30, 37–49. [Google Scholar] [CrossRef]
- Takács, Z.; Poór, P.; Borbély, P.; Czékus, Z.; Szalai, G.; Tari, I. H2O2 Homeostasis in Wild-Type and Ethylene-Insensitive Never Ripe Tomato in Response to Salicylic Acid Treatment in Normal Photoperiod and in Prolonged Darkness. Plant Physiol. Biochem. 2018, 126, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Yang, C.Y. Ethylene-Mediated Signaling Confers Thermotolerance and Regulates Transcript Levels of Heat Shock Factors in Rice Seedlings under Heat Stress. Bot. Stud. 2019, 60, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, Z.; Zhang, H.; Wang, X.C.; Huang, R. Transcriptional Modulation of Ethylene Response Factor Protein JERF3 in the Oxidative Stress Response Enhances Tolerance of Tobacco Seedlings to Salt, Drought, and Freezing. Plant Physiol. 2008, 148, 1953–1963. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Skalák, J.; Cerný, M.; Jedelský, P.; Dobrá, J.; Ge, E.; Novák, J.; Hronková, M.; Dobrev, P.; Vanková, R.; Brzobohatý, B. Stimulation of Ipt Overexpression as a Tool to Elucidate the Role of Cytokinins in High Temperature Responses of Arabidopsis Thaliana. J. Exp. Bot. 2016, 67, 2861–2873. [Google Scholar] [CrossRef] [Green Version]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin Action in Response to Abiotic and Biotic Stresses in Plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef]
- Macková, H.; Hronková, M.; Dobrá, J.; Turečková, V.; Novák, O.; Lubovská, Z.; Motyka, V.; Haisel, D.; Hájek, T.; Prášil, I.T.; et al. Enhanced Drought and Heat Stress Tolerance of Tobacco Plants with Ectopically Enhanced Cytokinin Oxidase/Dehydrogenase Gene Expression. J. Exp. Bot. 2013, 64, 2805–2815. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Kramna, B.; Gaudinova, A.; Knirsch, V.; Spichal, L.; Zatloukal, M.; Vankova, R. Heat Acclimation and Inhibition of Cytokinin Degradation Positively Affect Heat Stress Tolerance of Arabidopsis. Front. Plant Sci. 2020, 11, 87. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms. Regulation 2009, 11, 861–905. [Google Scholar] [CrossRef] [Green Version]
- Bäurle, I. Plant Heat Adaptation: Priming in Response to Heat Stress. F1000Research 2016, 18, 694. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.C.; Kim, S.H.; Lee, K.; Kim, K.E.; Liu, X.M.; Han, H.J.; Hoang, M.H.T.; Lee, S.W.; Hong, J.C.; Moon, Y.H.; et al. Identification of a C2H2-Type Zinc Finger Transcription Factor (ZAT10) from Arabidopsis as a Substrate of MAP Kinase. Plant Cell Rep. 2012, 31, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Baker, A.; Wright, M.; Sparkes, I.A.; Mhamdi, A.; Schippers, J.H.M.; Van Breusegem, F. On the Move: Redox-Dependent Protein Relocation in Plants. J. Exp. Bot. 2020, 71, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.; Chibani, K.; Jacquot, J.P.; Rouhier, N. Cysteine-Based Redox Regulation and Signaling in Plants. Front. Plant Sci. 2013, 4, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadaro, D.; Yun, B.W.; Spoel, S.H.; Chu, C.; Wang, Y.Q.; Loake, G.J. The Redox Switch: Dynamic Regulation of Protein Function by Cysteine Modifications. Physiol. Plant. 2010, 138, 360–371. [Google Scholar] [CrossRef]
- Davies, M.J. The Oxidative Environment and Protein Damage. Biochim. Biophys. Acta Proteins Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of Hydrogen Peroxide and Nitric Oxide on Both Salt and Heat Stress Tolerance in Rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, W. Protein S-Nitrosylation in Plant Abiotic Stresses. Funct. Plant Biol. 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Song, L.; Ding, W.; Zhao, M.; Sun, B.; Zhang, L. Nitric Oxide Protects against Oxidative Stress under Heat Stress in the Calluses from Two Ecotypes of Reed. Plant Sci. 2006, 171, 449–458. [Google Scholar] [CrossRef]
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang, W.H. Nitric Reductase-Dependent Nitric Oxide Production Is Involved in Cold Acclimation and Freezing Tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Parankusam, S.; Adimulam, S.S.; Bhatnagar-Mathur, P.; Sharma, K.K. Nitric Oxide (NO) in Plant Heat Stress Tolerance: Current Knowledge and Perspectives. Front. Plant Sci. 2017, 8, 1582. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Chen, J.; EF, A.A.; Wang, P.; Wang, G.; Hu, X.; Shi, J. Quantitative Proteomics Analysis Reveals That S-Nitrosoglutathione Reductase (GSNOR) and Nitric Oxide Signaling Enhance Poplar Defense against Chilling Stress. Planta 2015, 242, 1361–1390. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sun, L.; Huang, J.; Göschl, C.; Shi, W.; Chory, J.; Busch, W. GSNOR Provides Plant Tolerance to Iron Toxicity via Preventing Iron-Dependent Nitrosative and Oxidative Cytotoxicity. Nat. Commun. 2019, 10, 3896. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.; Wie, C.; Fernandez, B.O.; Feelisch, M.; Vierling, E. Modulation of Nitrosative Stress by S-Nitrosoglutathione Reductase Is Critical for Thermotolerance and Plant Growth in Arabidopsis. Plant Cell 2008, 20, 786–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Guerra, D.; Lee, U.; Vierling, E. S-Nitrosoglutathione Reductases Are Low-Copy Number, Cysteine-Rich Proteins in Plants That Control Multiple Developmental and Defense Responses in Arabidopsis. Front. Plant Sci. 2013, 4, 430. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.; Ghesquière, B.; Van Breusegem, F.; Gevaert, K. Plant Proteins under Oxidative Attack. Proteomics 2013, 13, 932–940. [Google Scholar] [CrossRef]
- Ancín, M.; Millan, A.F.S.; Larraya, L.; Morales, F.; Veramendi, J.; Aranjuelo, I.; Farran, I. Overexpression of Thioredoxin m in Tobacco Chloroplasts Inhibits the Protein Kinase STN7 and Alters Photosynthetic Performance. J. Exp. Bot. 2019, 70, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Reiland, S.; Messerli, G.; Baerenfaller, K.; Gerrits, B.; Endler, A.; Grossmann, J.; Gruissem, W.; Baginsky, S. Large-Scale Arabidopsis Phosphoproteome Profiling Reveals Novel Chloroplast Kinase Substrates and Phosphorylation Networks1[W]. Plant Physiol. 2009, 150, 889–903. [Google Scholar] [CrossRef] [Green Version]
- Park, S.K.; Jung, Y.J.; Lee, J.R.; Lee, Y.M.; Jang, H.H.; Lee, S.S.; Park, J.H.; Kim, S.Y.; Moon, J.C.; Lee, S.Y.; et al. Heat-Shock and Redox-Dependent Functional Switching of an h-Type Arabidopsis Thioredoxin from a Disulfide Reductase to a Molecular Chaperone. Plant Physiol. 2009, 150, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, H.B.; Moon, J.C.; Shin, M.R.; Chi, Y.H.; Jung, Y.J.; Lee, S.Y.; Nawkar, G.M.; Jung, H.S.; Hyun, J.K.; Kim, W.Y.; et al. Thioredoxin Reductase Type C (NTRC) Orchestrates Enhanced Thermotolerance to Arabidopsis by Its Redox-Dependent Holdase Chaperone Function. Mol. Plant 2013, 6, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, M.E.; Lee, C. The Redox Switch That Regulates Molecular Chaperones. Biomol. Concepts 2015, 6, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Knuesting, J.; Bariat, L.; Dard, A.; Freibert, S.A.; Marchand, C.H.; Young, D.; Dung, N.H.T.; Voth, W.; Debures, A.; et al. Redox Modification of the Iron-Sulfur Glutaredoxin GRXS17 Activates Holdase Activity and Protects Plants from Heat Stress. Plant Physiol. 2020, 184, 676–692. [Google Scholar] [CrossRef]
- Paeng, S.K.; Chi, Y.H.; Kang, C.H.; Chae, H.B.; Lee, E.S.; Park, J.H.; Wi, S.D.; Bae, S.B.; Phan, K.A.T.; Lee, S.Y. Chaperone Function of Arabidopsis NPR1. Plant Biotechnol. Rep. 2020, 14, 227–233. [Google Scholar] [CrossRef]
- Moon, J.C.; Jang, H.H.; Chae, H.B.; Lee, J.R.; Lee, S.Y.; Jung, Y.J.; Shin, M.R.; Lim, H.S.; Chung, W.S.; Yun, D.J.; et al. The C-Type Arabidopsis Thioredoxin Reductase ANTR-C Acts as an Electron Donor to 2-Cys Peroxiredoxins in Chloroplasts. Biochem. Biophys. Res. Commun. 2006, 348, 478–484. [Google Scholar] [CrossRef]
- Pérez-Ruiz, J.M.; Spínola, M.C.; Kirchsteiger, K.; Moreno, J.; Sahrawy, M.; Cejudo, F.J. Rice NTRC Is a High-Efficiency Redox System for Chloroplast Protection against Oxidative Damage. Plant Cell 2006, 18, 2356. [Google Scholar] [CrossRef] [Green Version]
- Alkhalfioui, F.; Renard, M.; Vensel, W.H.; Wong, J.; Tanaka, C.K.; Hurkman, W.J.; Buchanan, B.B.; Montrichard, F. Thioredoxin-Linked Proteins Are Reduced during Germination of Medicago Truncatula Seeds. Plant Physiol. 2007, 144, 1559–1579. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ruiz, J.M.; Cejudo, F.J. A Proposed Reaction Mechanism for Rice NADPH Thioredoxin Reductase C, an Enzyme with Protein Disulfide Reductase Activity. FEBS Lett. 2009, 583, 1399–1402. [Google Scholar] [CrossRef] [Green Version]
- Wulff, R.P.; Lundqvist, J.; Rutsdottir, G.; Hansson, A.; Stenbaek, A.; Elmlund, D.; Elmlund, H.; Jensen, P.E.; Hansson, M. The Activity of Barley NADPH-Dependent Thioredoxin Reductase C Is Independent of the Oligomeric State of the Protein: Tetrameric Structure Determined by Cryo-Electron Microscopy. Biochemistry 2011, 50, 3713–3723. [Google Scholar] [CrossRef]
- Chae, H.Z.; Oubrahim, H.; Park, J.W.; Rhee, S.G.; Chock, P.B. Protein Glutathionylation in the Regulation of Peroxiredoxins: A Family of Thiol-Specific Peroxidases That Function As Antioxidants, Molecular Chaperones, and Signal Modulators. Antioxid. Redox Signal. 2012, 16, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, S.G.; Woo, H.A.; Kil, I.S.; Bae, S.H. Peroxiredoxin Functions as a Peroxidase and a Regulator and Sensor of Local Peroxides. J. Biol. Chem. 2012, 287, 4403–4410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Alam, I.; Lee, K.W.; Sharmin, S.A.; Kwak, S.S.; Lee, S.Y.; Lee, B.H. Enhanced Tolerance of Transgenic Tall Fescue Plants Overexpressing 2-Cys Peroxiredoxin against Methyl Viologen and Heat Stresses. Biotechnol. Lett. 2010, 32, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B.; Nelson, K.J. Discovering Mechanisms of Signaling-Mediated Cysteine Oxidation. Curr. Opin. Chem. Biol. 2008, 12, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Phalen, T.J.; Weirather, K.; Deming, P.B.; Anathy, V.; Howe, A.K.; Van Der Vliet, A.; Jönsson, T.J.; Poole, L.B.; Heintz, N.H. Oxidation State Governs Structural Transitions in Peroxiredoxin II That Correlate with Cell Cycle Arrest and Recovery. J. Cell Biol. 2006, 175, 779–789. [Google Scholar] [CrossRef]
- Sprague, S.A.; Tamang, T.M.; Steiner, T.; Wu, Q.; Hu, Y.; Kakeshpour, T.; Park, J.; Yang, J.; Peng, Z.; Bergkamp, B.; et al. Redox-Engineering Enhances Maize Thermotolerance and Grain Yield in the Field. Plant Biotechnol. J. 2022, 20, 1819–1832. [Google Scholar] [CrossRef]
- Birkenbihl, R.P.; Liu, S.; Somssich, I.E. Transcriptional Events Defining Plant Immune Responses. Curr. Opin. Plant Biol. 2017, 38, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Feng, L.; Li, J.; He, Z. Genetic and Epigenetic Control of Plant Heat Responses. Front. Plant Sci. 2015, 6, 267. [Google Scholar] [CrossRef]
- Zhu, J.-K. Active DNA Methylation Mediated by DNA Glycosylases The Interplay between Intragenic Heterochromatin and RNA Processing View Project Stomatal Development View Project. Artic. Annu. Rev. Genet. 2009, 43, 143–166. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Desmarais, T.L.; Tong, Z.; Yao, Y.; Costa, M. Oxidative Stress Alters Global Histone Modification and DNA Methylation. Free Radic. Biol. Med. 2015, 82, 22–28. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Van Breusegem, F.; Mhamdi, A. Redox-Dependent Control of Nuclear Transcription in Plants. J. Exp. Bot. 2018, 69, 3359–3372. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Gupta, D.S.; Kesari, R.; Verma, R.; Murugesan, S.; Basu, P.S.; Soren, K.R.; Gupta, S.; Singh, N.P. Comprehensive RNAseq Analysis for Identification of Genes Expressed under Heat Stress in Lentil. Physiol. Plant. 2021, 173, 1785–1807. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS Signaling: The New Wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Mittler, R. Could Heat Shock Transcription Factors Function as Hydrogen Peroxide Sensors in Plants? Ann. Bot. 2006, 98, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.K.; Tripp, J.; Winkelhaus, S.; Tschiersch, B.; Theres, K.; Nover, L.; Scharf, K.D. In the Complex Family of Heat Stress Transcription Factors, HsfA1 Has a Unique Role as Master Regulator of Thermotolerance in Tomato. Genes Dev. 2002, 16, 1555–1567. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Ohama, N.; Nakajima, J.; Kidokoro, S.; Mizoi, J.; Nakashima, K.; Maruyama, K.; Kim, J.M.; Seki, M.; Todaka, D.; et al. Arabidopsis HsfA1 Transcription Factors Function as the Main Positive Regulators in Heat Shock-Responsive Gene Expression. Mol. Genet. Genomics 2011, 286, 321–332. [Google Scholar] [CrossRef]
- Hübel, A.; Schöffl, F. Arabidopsis Heat Shock Factor: Isolation and Characterization of the Gene and the Recombinant Protein. Plant Mol. Biol. 1994, 26, 353–362. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Chen, J.; Guo, L.; Li, X.; Li, W.; Yu, Z.; Deng, J.; Zhang, P.; Zhang, K.; et al. Arabidopsis Heat Shock Factor HsfA1a Directly Senses Heat Stress, PH Changes, and Hydrogen Peroxide via the Engagement of Redox State. Plant Physiol. Biochem. 2013, 64, 92–98. [Google Scholar] [CrossRef]
- Xuan, Y.; Zhou, S.; Wang, L.; Cheng, Y.; Zhao, L. Nitric Oxide Functions as a Signal and Acts Upstream of AtCaM3 in Thermotolerance in Arabidopsis Seedlings. Plant Physiol. 2010, 153, 1895–1906. [Google Scholar] [CrossRef] [Green Version]
- Andrási, N.; Pettkó-Szandtner, A.; Szabados, L. Diversity of Plant Heat Shock Factors: Regulation, Interactions, and Functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef]
- Pérez-Salamó, I.; Papdi, C.; Rigó, G.; Zsigmond, L.; Vilela, B.; Lumbreras, V.; Nagy, I.; Horváth, B.; Domoki, M.; Darula, Z.; et al. The Heat Shock Factor A4A Confers Salt Tolerance and Is Regulated by Oxidative Stress and the Mitogen-Activated Protein Kinases MPK3 and MPK6. Plant Physiol. 2014, 165, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Andrási, N.; Rigó, G.; Zsigmond, L.; Pérez-Salamó, I.; Papdi, C.; Klement, E.; Pettkó-Szandtner, A.; Baba, A.I.; Ayaydin, F.; Dasari, R.; et al. The Mitogen-Activated Protein Kinase 4-Phosphorylated Heat Shock Factor A4A Regulates Responses to Combined Salt and Heat Stresses. J. Exp. Bot. 2019, 70, 4903–4918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, N.Y.; Chen, L.S.; Sun, A.Z.; Zhao, Y.; Yin, S.N.; Guo, F.Q. A Nitric Oxide Burst at the Shoot Apex Triggers a Heat-Responsive Pathway in Arabidopsis. Nat. Plants 2022, 8, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Giesguth, M.; Sahm, A.; Simon, S.; Dietz, K.J. Redox-Dependent Translocation of the Heat Shock Transcription Factor AtHSFA8 from the Cytosol to the Nucleus in Arabidopsis Thaliana. FEBS Lett. 2015, 589, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Knuesting, J.; Birkholz, O.; Heinisch, J.J.; Scheibe, R. Cytosolic GAPDH as a Redox-Dependent Regulator of Energy Metabolism. BMC Plant Biol. 2018, 18, 184. [Google Scholar] [CrossRef] [Green Version]
- Vescovi, M.; Zaffagnini, M.; Festa, M.; Trost, P.; Lo Schiavo, F.; Costa, A. Nuclear Accumulation of Cytosolic Glyceraldehyde-3-Phosphate Dehydrogenase in Cadmium-Stressed Arabidopsis Roots. Plant Physiol. 2013, 162, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, E.; Fung, N.; Liu, J.; Drakakaki, G.; Coaker, G. Beyond Glycolysis: GAPDHs Are Multi-Functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses. PLoS Genet. 2015, 11, e1005199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Wang, Y.; Zheng, X.; Jia, Q.; Zhao, J.; Bai, F.; Hong, Y.; Liu, Y. Cytoplastic Glyceraldehyde-3-Phosphate Dehydrogenases Interact with ATG3 to Negatively Regulate Autophagy and Immunity in Nicotiana Benthamiana. Plant Cell 2015, 27, 1316–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.C.; Guo, L.; Wang, X. Nuclear Moonlighting of Cytosolic Glyceraldehyde-3-Phosphate Dehydrogenase Regulates Arabidopsis Response to Heat Stress. Nat. Commun. 2020, 11, 3439. [Google Scholar] [CrossRef]
- Krause, K.; Kilbienski, I.; Mulisch, M.; Rödiger, A.; Schäfer, A.; Krupinska, K. DNA-Binding Proteins of the Whirly Family in Arabidopsis Thaliana Are Targeted to the Organelles. FEBS Lett. 2005, 579, 3707–3712. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, E.; Miao, Y.; Mulisch, M.; Krupinska, K. Single-Stranded DNA-Binding Protein Whirly1 in Barley Leaves Is Located in Plastids and the Nucleus of the Same Cell. Plant Physiol. 2008, 147, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappadocia, L.; Parent, J.S.; Zampini, É.; Lepage, É.; Sygusch, J.; Brisson, N. A Conserved Lysine Residue of Plant Whirly Proteins Is Necessary for Higher Order Protein Assembly and Protection against DNA Damage. Nucleic Acids Res. 2012, 40, 258–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupinska, K.; Oetke, S.; Desel, C.; Mulisch, M.; Schäfer, A.; Hollmann, J.; Kumlehn, J.; Hensel, G. WHIRLY1 Is a Major Organizer of Chloroplast Nucleoids. Front. Plant Sci. 2014, 5, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Lin, W.; Deng, B.; Ren, Y.; Miao, Y. Dual-Located WHIRLY1 Interacting with LHCA1 Alters Photochemical Activities of Photosystem i and Is Involved in Light Adaptation in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 2352. [Google Scholar] [CrossRef] [Green Version]
- Desveaux, D.; Subramaniam, R.; Després, C.; Mess, J.N.; Lévesque, C.; Fobert, P.R.; Dangl, J.L.; Brisson, N. A “Whirly” Transcription Factor Is Required for Salicylic Acid-Dependent Disease Resistance in Arabidopsis. Dev. Cell 2004, 6, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Desveaux, D.; Despres, C.; Joyeux, A.; Subramaniam, R.; Brisson, N. PBF-2 Is a Novel Single-Stranded DNA Binding Factor Implicated in PR-10a Gene Activation in Potato. Plant Cell 2000, 12, 1477. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, K.; Gao, Y.; Liu, Z.; Diao, P.; Sui, N.; Meng, Q.; Meng, C.; Kong, F. WHIRLY1 Regulates HSP21.5A Expression to Promote Thermotolerance in Tomato. Plant Cell Physiol. 2020, 61, 169–177. [Google Scholar] [CrossRef]
- Foyer, C.H.; Karpinska, B.; Krupinska, K. The Functions of WHIRLY1 and REDOXRESPONSIVE TRANSCRIPTION FACTOR 1 in Cross Tolerance Responses in Plants: A Hypothesis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Georgii, E.; Jin, M.; Zhao, J.; Kanawati, B.; Schmitt-Kopplin, P.; Albert, A.; Winkler, J.B.; Schäffner, A.R. Relationships between Drought, Heat and Air Humidity Responses Revealed by Transcriptome-Metabolome Co-Analysis. BMC Plant Biol. 2017, 17, 120. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and Unique Responses of Plants to Multiple Individual Stresses and Stress Combinations: Physiological and Molecular Mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-Morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Francesca, S.; Vitale, L.; Arena, C.; Raimondi, G.; Olivieri, F.; Cirillo, V.; Paradiso, A.; de Pinto, M.C.; Maggio, A.; Barone, A.; et al. The Efficient Physiological Strategy of a Novel Tomato Genotype to Adapt to Chronic Combined Water and Heat Stress. Plant Biol. 2022, 24, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 871, 1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]



| Protein | Uniprot Code | Structure and Function | References | |
|---|---|---|---|---|
| Physiological Conditions | Heat Stress | |||
| AtTrx-h3 (thioredoxin) | Q42403 | LMW complexes disulfide reductase activity. | HMW complexes molecular chaperone. | [195] |
| NTRC (thioredoxin reductase) | Q70G58 | LMW complexes disulfide reductase and foldase chaperone. | HMW complexes holdase chaperone. | [196] |
| 2-Cys Prx A (peroxiredoxin) | Q96291 | LMW complexes peroxidase activity. | HMW complexes chaperone. | [197] |
| GRXS17 (Monothiol glutaredoxin) | Q9ZPH2 | LMW complexes glutaredoxin involved in the maturation of Fe–S proteins. | HMW complexes chaperone with holdase activity. | [198] |
| AtNPR1 (Non-expressor of pathogenesis related proteins 1) | P93002 | Monomeric form positive regulator of plant systemic acquired response. | Oligomeric form chaperone. | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox Signaling in Plant Heat Stress Response. Antioxidants 2023, 12, 605. https://doi.org/10.3390/antiox12030605
Fortunato S, Lasorella C, Dipierro N, Vita F, de Pinto MC. Redox Signaling in Plant Heat Stress Response. Antioxidants. 2023; 12(3):605. https://doi.org/10.3390/antiox12030605
Chicago/Turabian StyleFortunato, Stefania, Cecilia Lasorella, Nunzio Dipierro, Federico Vita, and Maria Concetta de Pinto. 2023. "Redox Signaling in Plant Heat Stress Response" Antioxidants 12, no. 3: 605. https://doi.org/10.3390/antiox12030605
APA StyleFortunato, S., Lasorella, C., Dipierro, N., Vita, F., & de Pinto, M. C. (2023). Redox Signaling in Plant Heat Stress Response. Antioxidants, 12(3), 605. https://doi.org/10.3390/antiox12030605