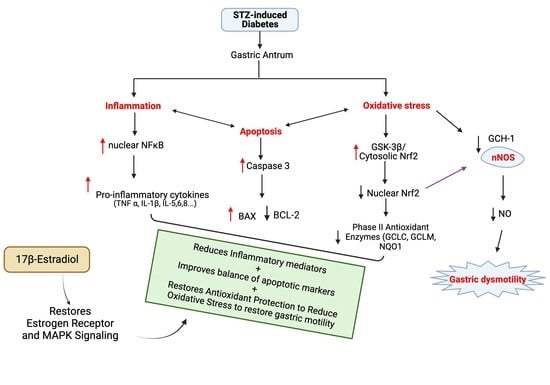
17β-Estradiol Suppresses Gastric Inflammatory and Apoptotic Stress Responses and Restores nNOS-Mediated Gastric Emptying in Streptozotocin (STZ)-Induced Diabetic Female Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design and STZ-Induced Diabetes
2.3. Measurement of Gastric Emptying
2.4. Neuromuscular Recording with Electric Field Stimulation
2.5. Evaluation of 17β-Estradiol, Insulin, MDA, IL-6, TNFα, IGF-1, and Total Nitrite Concentrations in Mouse Serum
2.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.7. Subcellular Fractionation
2.8. Gel Electrophoresis and Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. E2 Supplementation Normalized Body Weight, Blood Glucose Levels, Oxidative Stress Response, and Levels of Circulatory Inflammation Markers in STZ-Induced Diabetic Female Mice
3.2. E2 Supplementation Restored Gastric Emptying and Nitrergic Relaxation in STZ-Induced Diabetic Female Mice
3.3. E2 Supplementation Affected ERs and MAPK mRNA and Protein Levels in STZ-Induced Diabetic Female Mice
3.4. E2 Supplementation Restored the Levels of GSK-3β, Cytosolic and Nuclear Nrf2, and Phase II Antioxidant Enzymes to Normal Levels in STZ-Treated Diabetic Female Mice
3.5. E2 Supplementation Normalized the Levels of Gastric nNOSα and GCH-1 Proteins in Diabetic Mice
3.6. E2 supplementation Restored Levels of Nuclear NFκB and Gastric Pro-Inflammatory Cytokines in STZ-Treated Diabetic Mice to Levels Comparable to Those in Healthy Mice
3.7. E2 Supplementation Affected the Expression of Apoptotic Markers Bax, BCL-2, and Caspase 3 in STZ-Induced Diabetic Female Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanculete, M.F.; Chiarioni, G.; Dumitrascu, D.L.; Dumitrascu, D.I.; Popa, S.-L. Disorders of the brain-gut interaction and eating disorders. World J. Gastroenterol. 2021, 27, 3668–3681. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, S.; Abell, T.L. Diabetic Gastroparesis: Principles and Current Trends in Management. Diabetes Ther. 2018, 9, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharucha, A.E.; Kudva, Y.C.; Prichard, D.O. Diabetic Gastroparesis. Endocr. Rev. 2019, 40, 1318–1352. [Google Scholar] [CrossRef]
- Idrizaj, E.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Nitric Oxide: From Gastric Motility to Gastric Dysmotility. Int. J. Mol. Sci. 2021, 22, 9990. [Google Scholar] [CrossRef] [PubMed]
- Al-Shboul, O.; Nazzal, M.; Mustafa, A.; Al-Dwairi, A.; Alqudah, M.; Abu Omar, A.; Alfaqih, M.; Alsalem, M. Estrogen relaxes gastric muscle cells via a nitric oxide- and cyclic guanosine monophosphate-dependent mechanism: A sex-associated differential effect. Exp. Ther. Med. 2018, 16, 1685–1692. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Chapman, A.; Javed, S.; Alam, U.; Malik, R.A.; Azmi, S. The Investigation and Treatment of Diabetic Gastroparesis. Clin. Ther. 2018, 40, 850–861. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Loganathan, P.; Sarosiek, I.; McCallum, R.W. Gender-Related Differences in Gastroparesis. Am. J. Med. Sci. 2020, 360, 474–483. [Google Scholar] [CrossRef]
- Gangula, P.R.R.; Sekhar, K.R.; Mukhopadhyay, S. Gender bias in gastroparesis: Is nitric oxide the answer? Dig. Dis. Sci. 2011, 56, 2520–2527. [Google Scholar] [CrossRef] [Green Version]
- Dickman, R.; Wainstein, J.; Glezerman, M.; Niv, Y.; Boaz, M. Gender aspects suggestive of gastroparesis in patients with diabetes mellitus: A cross-sectional survey. BMC Gastroenterol. 2014, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Mori, H.; Suzuki, H.; Matsuzaki, J.; Taniguchi, K.; Shimizu, T.; Yamane, T.; Masaoka, T.; Kanai, T. Gender Difference of Gastric Emptying in Healthy Volunteers and Patients with Functional Dyspepsia. Digestion 2017, 95, 72–78. [Google Scholar] [CrossRef]
- Ye, Y.; Yin, Y.; Huh, S.Y.; Almansa, C.; Bennett, D.; Camilleri, M. Epidemiology, Etiology, and Treatment of Gastroparesis: Real-World Evidence From a Large US National Claims Database. Gastroenterology 2022, 162, 109–121.e5. [Google Scholar] [CrossRef] [PubMed]
- Zia, J.K.; Heitkemper, M.M. Upper Gastrointestinal Tract Motility Disorders in Women, Gastroparesis, and Gastroesophageal Reflux Disease. Gastroenterol. Clin. N. Am. 2016, 45, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.; Orvar, K.B.; Theodórs, A.; Kjeld, M. The relationship of gastrointestinal symptoms and menstrual cycle phase in young healthy women. Laeknabladid 2006, 92, 677–682. [Google Scholar] [PubMed]
- Kashyap, P.; Farrugia, G. Oxidative stress: Key player in gastrointestinal complications of diabetes. Neurogastroenterol. Motil. 2011, 23, 111–114. [Google Scholar] [CrossRef]
- Petri, M.; Singh, I.; Baker, C.; Underkofler, C.; Rasouli, N. Diabetic gastroparesis: An overview of pathogenesis, clinical presentation and novel therapies, with a focus on ghrelin receptor agonists. J. Diabetes Complicat. 2021, 35, 107733. [Google Scholar] [CrossRef]
- Sprouse, J.C.; Sampath, C.; Gangula, P.R. Supplementation of 17β-Estradiol Normalizes Rapid Gastric Emptying by Restoring Impaired Nrf2 and nNOS Function in Obesity-Induced Diabetic Ovariectomized Mice. Antioxidants 2020, 9, 582. [Google Scholar] [CrossRef]
- Sampath, C.; Sprouse, J.C.; Freeman, M.L.; Gangula, P.R. Activation of Nrf2 attenuates delayed gastric emptying in obesity induced diabetic (T2DM) female mice. Free Radic. Biol. Med. 2019, 135, 132–143. [Google Scholar] [CrossRef]
- Sampath, C.; Wilus, D.; Tabatabai, M.; Freeman, M.L.; Gangula, P.R. Mechanistic role of antioxidants in rescuing delayed gastric emptying in high fat diet induced diabetic female mice. Biomed. Pharmacother. 2021, 137, 111370. [Google Scholar] [CrossRef]
- Sampath, C.; Raju, A.V.; Freeman, M.L.; Srinivasan, S.; Gangula, P.R. Nrf2 attenuates hyperglycemia-induced nNOS impairment in adult mouse primary enteric neuronal crest cells and normalizes stomach function. Am. J. Physiol. Liver Physiol. 2022, 322, G368–G382. [Google Scholar] [CrossRef]
- Ravella, K.; Al-Hendy, A.; Sharan, C.; Hale, A.B.; Channon, K.M.; Srinivasan, S.; Gangula, P.R. Chronic estrogen deficiency causes gastroparesis by altering neuronal nitric oxide synthase function. Dig. Dis. Sci. 2013, 58, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, M.; Fichna, J.; Bashashati, M.; Habibi, S.; Sibaev, A.; Timmermans, J.-P.; Storr, M. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol. Motil. 2017, 29, e13025. [Google Scholar] [CrossRef] [Green Version]
- Shakhatreh, M.; Jehangir, A.; Malik, Z.; Parkman, H.P. Metoclopramide for the treatment of diabetic gastroparesis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.N. Estrogens and Gastroparesis: A Clinical Relevance. Dig. Dis. Sci. 2013, 58, 1449–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wu, G.-J.; Tang, Y.-R.; Lin, L. Role of estrogen in diabetic gastroparesis. World Chin. J. Dig. 2015, 23, 3888. [Google Scholar] [CrossRef]
- Lovick, T.A.; Zangrossi, H. Effect of Estrous Cycle on Behavior of Females in Rodent Tests of Anxiety. Front. Psychiatry 2021, 12, 711065. [Google Scholar] [CrossRef] [PubMed]
- Faltas, C.L.; LeBron, K.A.; Holz, M.K. Unconventional Estrogen Signaling in Health and Disease. Endocrinology 2020, 161, bqaa030. [Google Scholar] [CrossRef] [PubMed]
- Modder, U.; Riggs, B.; Spelsberg, T.; Fraser, D.; Atkinson, E.; Arnold, R.; Khosla, S. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur. J. Endocrinol. 2004, 151, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, M.M.; Eldridge, J.C.; Samson, H.H. Determination of an Estradiol Dose-Response Relationship in the Modulation of Ethanol Intake. Alcohol. Clin. Exp. Res. 2004, 28, 20–28. [Google Scholar] [CrossRef]
- Wan Mohammad, W.M.Z. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malaysian J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1. [Google Scholar] [CrossRef] [PubMed]
- Ingberg, E.; Theodorsson, E.; Theodorsson, A.; Ström, J.O. Effects of high and low 17β-estradiol doses on focal cerebral ischemia in rats. Sci. Rep. 2016, 6, 20228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Ming, W.; Li, Y.; Zheng, H.; Wei, C.; Rui, Z.; Yan, C. Synergistic Effect of a Physiological Ratio of Estradiol and Testosterone in the Treatment of Early-stage Atherosclerosis. Arch. Med. Res. 2015, 46, 619–629. [Google Scholar] [CrossRef]
- Sampath, C.; Srinivasan, S.; Freeman, M.L.; Gangula, P.R. Inhibition of GSK-3β restores delayed gastric emptying in obesity-induced diabetic female mice. Am. J. Physiol. Liver Physiol. 2020, 319, G481–G493. [Google Scholar] [CrossRef]
- Sprouse, J.; Sampath, C.; Gangula, P.R. Role of sex hormones and their receptors on gastric Nrf2 and neuronal nitric oxide synthase function in an experimental hyperglycemia model. BMC Gastroenterol. 2020, 20, 313. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Kügler, S.; Lastres-Becker, I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018, 14, 522–534. [Google Scholar] [CrossRef]
- Culbreth, M.; Aschner, M. GSK-3β, a double-edged sword in Nrf2 regulation: Implications for neurological dysfunction and disease. F1000Research 2018, 7, 1043. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [Green Version]
- Parsons, S.P.; Huizinga, J.D. Nitric Oxide Is Essential for Generating the Minute Rhythm Contraction Pattern in the Small Intestine, Likely via ICC-DMP. Front. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- Beck, K.; Friebe, A.; Voussen, B. Nitrergic signaling via interstitial cells of Cajal and smooth muscle cells influences circular smooth muscle contractility in murine colon. Neurogastroenterol. Motil. 2018, 30, e13300. [Google Scholar] [CrossRef] [PubMed]
- Heine, C.L.; Kolesnik, B.; Schmidt, R.; Werner, E.R.; Mayer, B.; Gorren, A.C.F. Interaction between Neuronal Nitric-Oxide Synthase and Tetrahydrobiopterin Revisited: Studies on the Nature and Mechanism of Tight Pterin Binding. Biochemistry 2014, 53, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Bagchi, A. NFkB pathway and inhibition: An overview. Comput. Mol. Biol. 2016, 6, 1–20. [Google Scholar] [CrossRef]
- Herring, B.P.; Chen, M.; Mihaylov, P.; Hoggatt, A.M.; Gupta, A.; Nakeeb, A.; Choi, J.N.; Wo, J.M. Transcriptome profiling reveals significant changes in the gastric muscularis externa with obesity that partially overlap those that occur with idiopathic gastroparesis. BMC Med. Genom. 2019, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Quobatari, A.; Shangguan, Y.; Hong, S.; Wiley, J.W.; Quobatari, A. Diabetic autonomic neuropathy: Evidence for apoptosis in situ in the rat. Neurogastroenterol. Motil. 2004, 16, 335–345. [Google Scholar] [CrossRef]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, S.H.; Goudarzvand, M.; Neishabouri, S.H. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015, 15, 55. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Xu, J.; Lamm, V.; Vachaparambil, C.T.; Chen, H.; Cai, Q. Diabetic Gastroparesis and Nondiabetic Gastroparesis. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 15–25. [Google Scholar] [CrossRef]
- Ohiagu, F.O.; Chikezie, P.C.; Chikezie, C.M. Pathophysiology of diabetes mellitus complications: Metabolic events and control. Biomed. Res. Ther. 2021, 8, 4243–4257. [Google Scholar] [CrossRef]
- Zheng, H.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.; Zhang, D.D. Therapeutic Potential of Nrf2 Activators in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Crimmins, S.; Smiley, R.; Preston, K.; Yau, A.; Mccallum, R.; Ali, M.S. Increased Expression of Pyloric ERβ Is Associated With Diabetic Gastroparesis in Streptozotocin-Induced Male Diabetic Rats. Gastroenterol. Res. 2016, 9, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Smiley, R.; Naik, P.; McCallum, R.; Showkat Ali, M. Reactive oxygen species overproduction and MAP kinase phosphatase-1 degradation are associated with gastroparesis in a streptozotocin-induced male diabetic rat model. Neurogastroenterol. Motil. 2018, 30, e13218. [Google Scholar] [CrossRef]
- Hatch, J.M.; Segvich, D.M.; Kohler, R.; Wallace, J.M. Skeletal manifestations in a streptozotocin-induced C57BL/6 model of Type 1 diabetes. Bone Rep. 2022, 17, 101609. [Google Scholar] [CrossRef] [PubMed]
- Thong, E.P.; Codner, E.; Laven, J.S.E.; Teede, H. Diabetes: A metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 2020, 8, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Khandekar, N.; Tong, S.; Yang, H.; Wang, W.; Huang, X.; Song, Z.; Lin, S. Reduced serum levels of oestradiol and brain derived neurotrophic factor in both diabetic women and HFD-feeding female mice. Endocrine 2017, 56, 65–72. [Google Scholar] [CrossRef]
- Wells, C.C.; Riazi, S.; Mankhey, R.W.; Bhatti, F.; Ecelbarger, C.; Maric, C. Diabetic nephropathy is associated with decreasedcirculating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend. Med. 2005, 2, 227–237. [Google Scholar] [CrossRef]
- Ziller, N.; Kotolloshi, R.; Esmaeili, M.; Liebisch, M.; Mrowka, R.; Baniahmad, A.; Liehr, T.; Wolf, G.; Loeffler, I. Sex Differences in Diabetes- and TGF-β1-Induced Renal Damage. Cells 2020, 9, 2236. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Gavin, K.M.; Seals, D.R.; Silver, A.E.; Moreau, K.L. Vascular Endothelial Estrogen Receptor α Is Modulated by Estrogen Status and Related to Endothelial Function and Endothelial Nitric Oxide Synthase in Healthy Women. J. Clin. Endocrinol. Metab. 2009, 94, 3513–3520. [Google Scholar] [CrossRef] [Green Version]
- Majmudar, N.G.; Robson, S.C.; Ford, G.A. Effects of the Menopause, Gender, and Estrogen Replacement Therapy on Vascular Nitric Oxide Activity. J. Clin. Endocrinol. Metab. 2000, 85, 1577–1583. [Google Scholar] [CrossRef]
- Yu, L.; Moore, A.B.; Castro, L.; Gao, X.; Huynh, H.-L.C.; Klippel, M.; Flagler, N.D.; Lu, Y.; Kissling, G.E.; Dixon, D. Estrogen Regulates MAPK-Related Genes through Genomic and Nongenomic Interactions between IGF-I Receptor Tyrosine Kinase and Estrogen Receptor-Alpha Signaling Pathways in Human Uterine Leiomyoma Cells. J. Signal Transduct. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Maier, B.; Santen, R.J.; Song, R.X.-D. Membrane association of estrogen receptor α mediates estrogen effect on MAPK activation. Biochem. Biophys. Res. Commun. 2002, 294, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, M.; Yang, L.; Zhang, L.; Guo, H.; Qin, P.; Hou, W. Loss of Estrogen Efficacy Against Hippocampus Damage in Long-Term OVX Mice Is Related to the Reduction of Hippocampus Local Estrogen Production and Estrogen Receptor Degradation. Mol. Neurobiol. 2020, 57, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- Ansarin, K.; Khoubnasabjafari, M.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 2017, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Pandey, R.; Shukla, P.; Anjum, B.; Gupta, H.P.; Pal, S.; Arjaria, N.; Gupta, K.; Chattopadhyay, N.; Sinha, R.A.; Bandyopadhyay, S. Estrogen deficiency induces memory loss via altered hippocampal HB-EGF and autophagy. J. Endocrinol. 2020, 244, 53–70. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef]
- Azarova, I.; Klyosova, E.; Lazarenko, V.; Konoplya, A.; Polonikov, A. Genetic variants in glutamate cysteine ligase confer protection against type 2 diabetes. Mol. Biol. Rep. 2020, 47, 5793–5805. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Jiang, J.-Z.; Cai, Y.-L.; Piao, L.-H.; Jin, Z. Significance of dynamic changes in gastric smooth muscle cell apoptosis, PI3K-AKT-mTOR and AMPK-mTOR signaling in a rat model of diabetic gastroparesis. Mol. Med. Rep. 2017, 16, 1530–1536. [Google Scholar] [CrossRef] [Green Version]
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Fang, X.; Cui, X.; Jin, Z. Mechanism of AMPK-mediated apoptosis of rat gastric smooth muscle cells under high glucose condition. Biosci. Rep. 2019, 39, BSR20192504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, C.; Zanner, R.; Rad, R.; Presecan-Siedel, E.; Schepp, W.; Classen, M.; Prinz, C. TNF-a-associated apoptosis in rat gastric enterochromaffin-like cells is mediated by NFKB and can be antagonized by BFGF. Gastroenterology 2000, 118, A888. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domazetovic, V.; Falsetti, I.; Ciuffi, S.; Iantomasi, T.; Marcucci, G.; Vincenzini, M.T.; Brandi, M.L. Effect of Oxidative Stress-Induced Apoptosis on Active FGF23 Levels in MLO-Y4 Cells: The Protective Role of 17-β-Estradiol. Int. J. Mol. Sci. 2022, 23, 2103. [Google Scholar] [CrossRef] [PubMed]
- Nennig, S.E.; Schank, J.R. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeño, J.M.; Fernandez-Delgado, R.; Fett, C.; Castaño-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-κB-Mediated Inflammation in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice Increases Survival. J. Virol. 2014, 88, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Sankari, S.; Babu, N.; Rajesh, E.; Kasthuri, M. Apoptosis in immune-mediated diseases. J. Pharm. Bioallied Sci. 2015, 7, 200. [Google Scholar] [CrossRef]
- Seabrook, N.; Kedar, A.; Bills, G.; Sarker, S.; Rock, W.A.; Pinkston, C.; Kedar, A.; Abell, T. Inflammatory Markers and Mortality in Diabetic Versus Idiopathic Gastroparesis. Am. J. Med. Sci. 2022, 363, 218–223. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Hamzawy, M.A.; El-Denshary, E.S.M.; Bahgat, A.K.; Hassan, N.S.; Aly, S.E.; Abdel-Wahhab, M.A. Hepatoprotective Effect of Estradiol and A-Lipoic Acid in Rats Article Application of Hazard Analysis Critical Control Points in Food Prosscing View Project. Glob. J. Pharmacol. 2014, 8, 694–704. [Google Scholar]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver-Williams, C.; Glisic, M.; Shahzad, S.; Brown, E.; Pellegrino Baena, C.; Chadni, M.; Chowdhury, R.; Franco, O.H.; Muka, T. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: A systematic review. Hum. Reprod. Update 2019, 25, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Mirkin, S.; Bernick, B.; Constantine, G.D. Systemic estradiol levels with low-dose vaginal estrogens. Menopause 2020, 27, 361–370. [Google Scholar] [CrossRef] [PubMed]








| Gene | Forward | Reverse | Accession Number |
|---|---|---|---|
| nNOS α | 5′-CCCAACGTCATTTCTGTCCGT-3′ | 5′-TCTACCAGGGGCCGATCATT-3′ | NM_008712 |
| ER α | 5′-CCCGCCTTCTACAGGTCTAAT-3′ | 5′-CTTTCTCGTTACTGCTGGACAG-3′ | NM_007956 |
| ER β | 5′-CTGTGATGAACTACAGTGTTCCC-3′ | 5′-CACATTTGGGCTTGCAGTCTG-3′ | NM_207707 |
| GCH-1 | 5′-GAGCATCACCTTGTTCCATTTG-3′ | 5′-GCCAAGTTTACTGAGACCAAGGA-3′ | NM_008102 |
| β-actin | 5′TGGAATCCTGTGGCATCCATGAAAC-3′ | 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ | NM_007393 |
| Nrf2 | 5′-TCTCCTCGCTGGAAAAAGAA-3′ | 5′-TAAAGCACAGCCAGCACATT-3′ | NM_010902 |
| GCLM | 5′-GCCCGCTCGCCATCTCTC-3′ | 5′-GTTGAGCAGGTTCCCGGTCT-3′ | NM_008129 |
| GCLC | 5′-ATGTGGACACCCGATGCAGTATT-3′ | 5′-TGTCTTGCTTGTAGTCAGGATGGTTT-3′ | NM_010295 |
| Nqo1 | 5′-GCCGAACACAAGAAGCTGGAAG-3′ | 5′-GGCAAATCCTGCTACGAGCACT-3′ | NM_008706 |
| P38MAPK | 5′-AGGGCGATGTGACGTTT-3′ | 5′-CTGGCAGGGTGAAGTTGG-3′ | NM_001168508 |
| GSK-3β | 5′-GCATTTATCATTAACCTAGCACCC-3′ | 5′-ATTTTCTTTCCAAACGTGACC-3′ | NM_019827 |
| IL 1α | 5′-ACGGCTGAGTTTCAGTGAGACC-3′ | 5′-CACTCTGGTAGGTGTAAGGTGC-3′ | NM_010554 |
| IL 1β | 5′-TGGACCTTCCAGGATGAGGACA-3′ | 5′-GTTCATCTCGGAGCCTGTAGTG-3′ | NM_008361 |
| IL 3 | 5′-CCTGCCTACATCTGCGAATGAC-3′ | 5′-GAGGTTAGCACTGTCTCCAGATC-3′ | NM_010556 |
| IL 5 | 5′-GATGAGGCTTCCTGTCCCTACT-3′ | 5′-TGACAGGTTTTGGAATAGCATTTCC-3′ | NM_010558 |
| IL 6 | 5′-TACCACTTCACAAGTCGGAGGC-3′ | 5′-CTGCAAGTGCATCATCGTTGTTC-3′ | NM_031168 |
| IL 8 | 5′-GAGAGTGATTGAGAGTGGACCAC-3 | 5′-CACAACCCTCTGCACCCAGTTT-3′ | NM_000584 |
| IL 11 | 5′-CTGACGGAGATCACAGTCTGGA-3′ | 5′-GGACATCAAGTCTACTCGAAGCC-3′ | NM_001290423 |
| IL 13 | 5′-AACGGCAGCATGGTATGGAGTG-3′ | 5′-TGGGTCCTGTAGATGGCATTGC-3′ | NM_008355 |
| IL 33 | 5′-CTACTGCATGAGACTCCGTTCTG-3′ | 5′-AGAATCCCGTGGATAGGCAGAG-3′ | NM_001164724 |
| TNF α | 5′-GGTGCCTATGTCTCAGCCTCTT-3′ | 5′-GCCATAGAACTGATGAGAGGGAG-3′ | NM_001278601 |
| Control | STZ | STZ + E2 (0.001 mg/Kg) | STZ + E2 (0.005 mg/Kg) | STZ + E2 (0.25 mg/Kg) | STZ + E2 (1.0 mg/Kg) | |
|---|---|---|---|---|---|---|
| Body weight (g) | 22.9 ± 0.5 | 18.8 ± 0.9 | 18.3 ± 0.8 | 19.0 ± 0.6 | 20.3 ± 0.8 | 19.7 ± 1.0 |
| Blood glucose (mg/DL) | 106 ± 07 | 441 ± 38 a | 446 ± 38 | 421 ± 24 | 324 ± 17 b | 389 ± 42 b |
| Serum Insulin (ng/mL) | 0.44 ± 0.04 | 0.24 ± 0.03 | 0.25 ±0.05 | 0.28 ± 0.04 | 0.31 ± 0.04 | 0.28 ± 0.05 |
| Serum nitrate (µM) | 31.5 ± 4.1 | 20.1 ± 3.3 a | 20.5 ± 3.1 | 20.8 ± 2.2 | 28.5 ± 3.5 b | 25.6 ± 2.1 b |
| Serum Estradiol (ng/L) | 33.4 ± 3.3 | 20.7 ± 2.8 a | 23.2 ± 3.4 | 25.6 ± 4.5 b | 34.8 ± 2.7 b | 43.6 ± 5.1 b |
| Serum MDA (nmol/mg protein) | 14 ± 2.4 | 42 ± 5.5 a | 40 ± 4.8 | 41 ± 5.1 | 20 ± 3.7 b | 28 ± 5.1 b |
| Serum IL-6 (ng/mL) | 88 ± 6 | 435 ± 32 a | 414 ± 28 | 404 ± 35 | 136 ± 15 b | 221 ± 44 b |
| Serum TNF (ng/mL) | 6 ± 0.6 | 22 ± 3 a | 21 ± 1.5 | 18 ± 2.4 | 10 ± 2.8 b | 14 ± 1.6 b |
| Serum IGF-1 (ng/mL) | 12 ± 1.2 | 33 ± 3.5 a | 30 ± 2.2 | 26 ± 3.1 | 15 ± 1.8 b | 22 ± 2.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprouse, J.; Sampath, C.; Gangula, P. 17β-Estradiol Suppresses Gastric Inflammatory and Apoptotic Stress Responses and Restores nNOS-Mediated Gastric Emptying in Streptozotocin (STZ)-Induced Diabetic Female Mice. Antioxidants 2023, 12, 758. https://doi.org/10.3390/antiox12030758
Sprouse J, Sampath C, Gangula P. 17β-Estradiol Suppresses Gastric Inflammatory and Apoptotic Stress Responses and Restores nNOS-Mediated Gastric Emptying in Streptozotocin (STZ)-Induced Diabetic Female Mice. Antioxidants. 2023; 12(3):758. https://doi.org/10.3390/antiox12030758
Chicago/Turabian StyleSprouse, Jeremy, Chethan Sampath, and Pandu Gangula. 2023. "17β-Estradiol Suppresses Gastric Inflammatory and Apoptotic Stress Responses and Restores nNOS-Mediated Gastric Emptying in Streptozotocin (STZ)-Induced Diabetic Female Mice" Antioxidants 12, no. 3: 758. https://doi.org/10.3390/antiox12030758
APA StyleSprouse, J., Sampath, C., & Gangula, P. (2023). 17β-Estradiol Suppresses Gastric Inflammatory and Apoptotic Stress Responses and Restores nNOS-Mediated Gastric Emptying in Streptozotocin (STZ)-Induced Diabetic Female Mice. Antioxidants, 12(3), 758. https://doi.org/10.3390/antiox12030758