Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation
Abstract
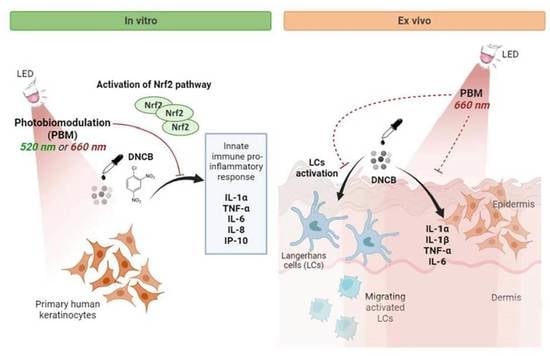
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Chemical Treatment
2.3. Light Source and Photobiomodulation Treatment
2.4. Gene Silencing
2.5. RNA Isolation and RT-qPCR
2.6. Protein Extraction and Western Blotting
2.7. Measurement of Nrf2 Transcription Factor Activity (DNA Binding)
2.8. Cytokines Dosage
2.9. Human Skin Explants Preparation and Treatment
2.10. Immunostaining of Skin Explants
2.11. Statistical Analysis
3. Results
3.1. PBM Regulates Pro-Inflammatory Mediators in Stressed KCs
3.2. PBM Enhances Nrf2 Pathway Activation in DNCB-Stimulated KCs
3.3. Nrf2 Mediates the Anti-Inflammatory Response of the Red Light in KCs
3.4. PBM Reduces DNCB-Induced LCs Activation and TNF-α Production in the Epidermis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Chapter 1-Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 1–14. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The Diverse Contribution of Keratinocytes to Immune Responses in Skin. JCI Insight 2020, 5, e1420672020. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous Barriers and Skin Immunity: Differentiating A Connected Network. Trends Immunol. 2018, 39, 315–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin Immune Sentinels in Health and Disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [Green Version]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms Regulating Skin Immunity and Inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Korkina, L.; Pastore, S. The Role of Redox Regulation in the Normal Physiology and Inflammatory Diseases of Skin. Front. Biosci. 2009, 1, 123–141. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, e5698931. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-e20. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Cha, Y.-N.; Surh, Y.-J. A Protective Role of Nuclear Factor-Erythroid 2-Related Factor-2 (Nrf2) in Inflammatory Disorders. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Stress-Sensing Mechanisms and the Physiological Roles of the Keap1–Nrf2 System during Cellular Stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An Overview of the Advantages of KEAP1-NRF2 System Activation During Inflammatory Disease Treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Nrf2 Involvement in Chemical-Induced Skin Innate Immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Ali, Z.; Gerbeix, C.; Hemon, P.; Esser, P.R.; Martin, S.F.; Pallardy, M.; Kerdine-Römer, S. Allergic Skin Inflammation Induced by Chemical Sensitizers Is Controlled by the Transcription Factor Nrf2. Toxicol. Sci. 2013, 134, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Vallion, R.; Hardonnière, K.; Bouredji, A.; Damiens, M.-H.; Deloménie, C.; Pallardy, M.; Ferret, P.-J.; Kerdine-Römer, S. The Inflammatory Response in Human Keratinocytes Exposed to Cinnamaldehyde Is Regulated by Nrf2. Antioxidants 2022, 11, 575. [Google Scholar] [CrossRef]
- Vallion, R.; Kerdine-Römer, S. Regulation of the Immune Response to Contact Sensitizers by Nrf2. Contact Dermat. 2022, 87, 13–19. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 Suppresses Macrophage Inflammatory Response by Blocking Pro-inflammatory Cytokine Transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [Green Version]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-Level Laser (Light) Therapy (LLLT) in Skin: Stimulating, Healing, Restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- Jagdeo, J.; Austin, E.; Mamalis, A.; Wong, C.; Ho, D.; Siegel, D.M. Light-emitting Diodes in Dermatology: A Systematic Review of Randomized Controlled Trials. Lasers Surg. Med. 2018, 50, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, F.F.; Simões, A.; Corrêa, L.; Aranha, A.C.C.; Giudice, F.S.; Hamblin, M.R.; Sousa, S.C.O.M. Low-Level Laser Irradiation Promotes the Proliferation and Maturation of Keratinocytes during Epithelial Wound Repair. J. Biophotonics 2015, 8, 795–803. [Google Scholar] [CrossRef] [Green Version]
- de Abreu, P.T.R.; de Arruda, J.A.A.; Mesquita, R.A.; Abreu, L.G.; Diniz, I.M.A.; Silva, T.A. Photobiomodulation Effects on Keratinocytes Cultured in Vitro: A Critical Review. Lasers Med. Sci. 2019, 34, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Multiple Roles of Cytochrome c Oxidase in Mammalian Cells under Action of Red and IR-A Radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lim, W.; Kim, I.; Kim, J.; Ko, Y.; Kwon, H.; Kim, S.; Kabir, K.M.A.; Li, X.; Kim, O.; et al. Inflammatory Cytokines Are Suppressed by Light-Emitting Diode Irradiation of P. Gingivalis LPS-Treated Human Gingival Fibroblasts: Inflammatory Cytokine Changes by LED Irradiation. Lasers Med. Sci. 2012, 27, 459–467. [Google Scholar] [CrossRef]
- Gupta, A.; Keshri, G.K.; Yadav, A.; Gola, S.; Chauhan, S.; Salhan, A.K.; Bala Singh, S. Superpulsed (Ga-As, 904 Nm) Low-Level Laser Therapy (LLLT) Attenuates Inflammatory Response and Enhances Healing of Burn Wounds. J. Biophotonics 2015, 8, 489–501. [Google Scholar] [CrossRef]
- Silveira, P.C.L.; Ferreira, K.B.; da Rocha, F.R.; Pieri, B.L.S.; Pedroso, G.S.; De Souza, C.T.; Nesi, R.T.; Pinho, R.A. Effect of Low-Power Laser (LPL) and Light-Emitting Diode (LED) on Inflammatory Response in Burn Wound Healing. Inflammation 2016, 39, 1395–1404. [Google Scholar] [CrossRef]
- de Farias Gabriel, A.; Wagner, V.P.; Correa, C.; Webber, L.P.; Pilar, E.F.S.; Curra, M.; Carrard, V.C.; Martins, M.A.T.; Martins, M.D. Photobiomodulation Therapy Modulates Epigenetic Events and NF-ΚB Expression in Oral Epithelial Wound Healing. Lasers Med. Sci. 2019, 34, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Curra, M.; Pellicioli, A.C.A.; Filho, N.A.K.; Ochs, G.; Matte, Ú.; Filho, M.S.; Martins, M.A.T.; Martins, M.D. Photobiomodulation Reduces Oral Mucositis by Modulating NF-KB. J. Biomed. Opt. 2015, 20, 125008. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Verma, S.; Keshri, G.K.; Gupta, A. Role of 904 Nm Superpulsed Laser-Mediated Photobiomodulation on Nitroxidative Stress and Redox Homeostasis in Burn Wound Healing. Photodermatol. Photoimmunol. Photomed. 2020, 36, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, C.; Declercq, L.; Decaux, G.; Grimaud, J.-A. Safety and Efficacy of a Novel Home-Use Device for Light-Potentiated (LED) Skin Treatment. J. Biophotonics 2020, 13, e202000230. [Google Scholar] [CrossRef] [PubMed]
- Clouet, E.; Bechara, R.; Raffalli, C.; Damiens, M.-H.; Groux, H.; Pallardy, M.; Ferret, P.-J.; Kerdine-Römer, S. The THP-1 Cell Toolbox: A New Concept Integrating the Key Events of Skin Sensitization. Arch. Toxicol. 2019, 93, 941–951. [Google Scholar] [CrossRef]
- de Bourayne, M.; Gallais, Y.; El Ali, Z.; Rousseau, P.; Damiens, M.-H.; Cochet, C.; Filhol, O.; Chollet-Martin, S.; Pallardy, M.; Kerdine-Römer, S. Protein Kinase CK2 Controls T-Cell Polarization through Dendritic Cell Activation in Response to Contact Sensitizers. J. Leukoc. Biol. 2017, 101, 703–715. [Google Scholar] [CrossRef]
- Patatian, A.; Deslestre-Delacour, C.; Percoco, G.; Ramdani, Y.; Di Giovanni, M.; Peno-Mazzarino, L.; Bader, T.; Bénard, M.; Driouich, A.; Lati, E.; et al. Skin Biological Responses to Urban Pollution in an Ex Vivo Model. Toxicol. Lett. 2021, 348, 85–96. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- West, H.C.; Bennett, C.L. Redefining the Role of Langerhans Cells As Immune Regulators within the Skin. Front. Immunol. 2017, 8, 1941. [Google Scholar] [CrossRef] [Green Version]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans Cells-Programmed by the Epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, M.; Narbutt, J.; Lesiak, A. Blue Light in Dermatology. Life 2021, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Scarponi, C.; Giustizieri, M.L.; Girolomoni, G. Keratinocytes in Inflammatory Skin Diseases. Curr. Drug Targets Inflamm. Allergy 2005, 4, 329–334. [Google Scholar] [CrossRef]
- Kimber, I.; Basketter, D.A.; Butler, M.; Gamer, A.; Garrigue, J.-L.; Gerberick, G.F.; Newsome, C.; Steiling, W.; Vohr, H.-W. Classification of Contact Allergens According to Potency: Proposals. Food Chem. Toxicol. 2003, 41, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Helou, D.G.; Noël, B.; Gaudin, F.; Groux, H.; El Ali, Z.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Cutting Edge: Nrf2 Regulates Neutrophil Recruitment and Accumulation in Skin during Contact Hypersensitivity. J. Immunol. 2019, 202, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, B.; Kim, J.-H.; Kim, E.-Y.; Kim, M.; Kwon, B.; Cho, H.-R.; Sohn, Y.; Jung, H.-S. Solanum Nigrum Linne Improves DNCB-induced Atopic Dermatitis-like Skin Disease in BALB/c Mice. Mol. Med. Rep. 2020, 22, 2878–2886. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hung, Y.-L.; Ko, W.-C.; Tsai, Y.-J.; Chang, J.-F.; Liang, C.-W.; Chang, D.-C.; Hung, C.-F. Effect of Neferine on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Int. J. Mol. Sci. 2021, 22, 8237. [Google Scholar] [CrossRef]
- Naik, S.M.; Cannon, G.; Burbach, G.J.; Singh, S.R.; Swerlick, R.A.; Ansel, J.C.; Caughman, S.W.; Wilcox, J.N. Human Keratinocytes Constitutively Express Interleukin-18 and Secrete Biologically Active Interleukin-18 After Treatment with Pro-Inflammatory Mediators and Dinitrochlorobenzene. J. Investig. Dermatol. 1999, 113, 766–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Byamba, D.; Wu, W.H.; Kim, T.-G.; Lee, M.-G. Different Characteristics of Reactive Oxygen Species Production by Human Keratinocyte Cell Line Cells in Response to Allergens and Irritants. Exp. Dermatol. 2012, 21, 99–103. [Google Scholar] [CrossRef]
- Oh, S.; Chung, H.; Chang, S.; Lee, S.-H.; Seok, S.H.; Lee, H. Effect of Mechanical Stretch on the DNCB-Induced Pro-inflammatory Cytokine Secretion in Human Keratinocytes. Sci. Rep. 2019, 9, 5156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, C.; Louafi, F.; McGuire, C.; Lowings, K.; Kumar, P.; Cooper, H.; Dearman, R.J.; Cumberbatch, M.; Kimber, I.; Healy, E.; et al. The Cutaneous Biochemical Redox Barrier: A Component of the Innate Immune Defenses against Sensitization by Highly Reactive Environmental Xenobiotics. J. Immunol. 2009, 183, 7576–7584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.L.; Lim, H.S.; Lee, S.M. Effect of Low-Level Laser Intervention on Dermatitis Symptoms and Cytokine Changes in DNCB-Induced Atopy Mouse Model: A Randomized Controlled Trial. Exp. Ther. Med. 2021, 22, 1196. [Google Scholar] [CrossRef] [PubMed]
- Catão, M.H.C.V.; Costa, R.O.; Nonaka, C.F.W.; Junior, R.L.C.A.; Costa, I.R.R.S. Green LED Light Has Anti-Inflammatory Effects on Burns in Rats. Burns 2016, 42, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Mitani, A.; Fukuda, M.; Mogi, M.; Osawa, K.; Takahashi, S.; Aino, M.; Iwamura, Y.; Miyajima, S.; Yamamoto, H.; et al. Irradiation with a Low-Level Diode Laser Induces the Developmental Endothelial Locus-1 Gene and Reduces Pro-inflammatory Cytokines in Epithelial Cells. Lasers Med. Sci. 2014, 29, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.P.S.; Souza, N.H.C.; Mesquita-Ferrari, R.A.; da Silva, D.D.F.T.; Rocha, L.A.; Alves, A.N.; de Brito Sousa, K.; Bussadori, S.K.; Hamblin, M.R.; Nunes, F.D. Photobiomodulation with 660-Nm and 780-Nm Laser on Activated J774 Macrophage-like Cells: Effect on M1 Inflammatory Markers. J. Photochem. Photobiol. B 2015, 153, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Sharma, M.R.; Mahendra, A.; Kumar, S. Role of Inflammatory Cytokines in Pathophysiology of Psoriasis. Curr. Pharmacol. Rep. 2022, 8, 99–105. [Google Scholar] [CrossRef]
- Becker, A.; Klapczynski, A.; Kuch, N.; Arpino, F.; Simon-Keller, K.; De La Torre, C.; Sticht, C.; van Abeelen, F.A.; Oversluizen, G.; Gretz, N. Gene Expression Profiling Reveals Aryl Hydrocarbon Receptor as a Possible Target for Photobiomodulation When Using Blue Light. Sci. Rep. 2016, 6, 33847. [Google Scholar] [CrossRef] [Green Version]
- Neves, L.M.S.; Gonçalves, E.C.D.; Cavalli, J.; Vieira, G.; Laurindo, L.R.; Simões, R.R.; Coelho, I.S.; Santos, A.R.S.; Marcolino, A.M.; Cola, M.; et al. Photobiomodulation Therapy Improves Acute Inflammatory Response in Mice: The Role of Cannabinoid Receptors/ATP-Sensitive K+ Channel/P38-MAPK Signalling Pathway. Mol. Neurobiol. 2018, 55, 5580–5593. [Google Scholar] [CrossRef]
- Kim, H.; Choi, M.S.; Bae, I.-H.; Jung, J.; Son, E.D.; Lee, T.R.; Shin, D.W. Short Wavelength Visible Light Suppresses Innate Immunity-Related Responses by Modulating Protein S-Nitrosylation in Keratinocytes. J. Investig. Dermatol. 2016, 136, 727–731. [Google Scholar] [CrossRef] [Green Version]
- Trotter, L.A.; Patel, D.; Dubin, S.; Guerra, C.; McCloud, V.; Lockwood, P.; Messer, R.; Wataha, J.C.; Lewis, J.B. Violet/Blue Light Activates Nrf2 Signaling and Modulates the Inflammatory Response of THP-1 Monocytes. Photochem. Photobiol. Sci. 2017, 16, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Rotenberg, S.; Messer, R.L.W.; Wataha, J.C.; Ogbureke, K.U.E.; Mccloud, V.V.; Lockwood, P.; Hsu, S.; Lewis, J.B. Blue Light Activates Phase 2 Response Proteins and Slows Growth of A431 Epidermoid Carcinoma Xenografts. Anticancer Res. 2014, 34, 6305–6313. [Google Scholar] [PubMed]
- Sohn, H.; Ko, Y.; Park, M.; Kim, D.; Moon, Y.L.; Jeong, Y.J.; Lee, H.; Moon, Y.; Jeong, B.-C.; Kim, O.; et al. Effects of Light-Emitting Diode Irradiation on RANKL-Induced Osteoclastogenesis. Lasers Surg. Med. 2015, 47, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, N.; Li, J.; Li, J.; Zhu, W.; Kuang, Y.; Chen, X.; Peng, C. The Role of Langerhans Cells in Epidermal Homeostasis and Pathogenesis of Psoriasis. J. Cell Mol. Med. 2020, 24, 11646–11655. [Google Scholar] [CrossRef]
- Ouwehand, K.; Santegoets, S.J.A.M.; Bruynzeel, D.P.; Scheper, R.J.; de Gruijl, T.D.; Gibbs, S. CXCL12 Is Essential for Migration of Activated Langerhans Cells from Epidermis to Dermis. Eur. J. Immunol. 2008, 38, 3050–3059. [Google Scholar] [CrossRef]
- Eaton, L.H.; Roberts, R.A.; Kimber, I.; Dearman, R.J.; Metryka, A. Skin Sensitization Induced Langerhans Cell Mobilization: Variable Requirements for Tumour Necrosis Factor-α. Immunology 2015, 144, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-S.; Chiu, H.-C.; Hsu, C.-J.; Liu, C.-Y.; Hsieh, P.-C.; Miaw, S.-C.; Yu, H.-S.; Wang, L.-F. Low-Energy Visible Light Irradiation Modulates Immune Responses Induced by Epicutaneous Sensitization with Protein Antigen. J. Investig. Dermatol. 2009, 129, 2258–2264. [Google Scholar] [CrossRef] [Green Version]
- Danno, K.; Sugie, N. Effects of Near-Infrared Radiation on the Epidermal Proliferation and Cutaneous Immune Function in Mice. Photodermatol. Photoimmunol. Photomed. 1996, 12, 233–236. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hong, C.-H.; Liao, W.-T.; Yu, H.-S. Differential Immunological Effects of Infrared Irradiation and Its Associated Heat in Vivo. J. Photochem. Photobiol. B Biol. 2016, 155, 98–103. [Google Scholar] [CrossRef]
- Yeang, H.X.A.; Hamdam, J.M.; Al-Huseini, L.M.A.; Sethu, S.; Djouhri, L.; Walsh, J.; Kitteringham, N.; Park, B.K.; Goldring, C.E.; Sathish, J.G. Loss of Transcription Factor Nuclear Factor-Erythroid 2 (NF-E2) P45-Related Factor-2 (Nrf2) Leads to Dysregulation of Immune Functions, Redox Homeostasis, and Intracellular Signaling in Dendritic Cells. J. Biol. Chem. 2012, 287, 10556–10564. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, S.; Guermonprez, C.; Peno-Mazzarino, L.; Lati, E.; Rousseaud, A.; Declercq, L.; Kerdine-Römer, S. Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation. Antioxidants 2023, 12, 766. https://doi.org/10.3390/antiox12030766
Salman S, Guermonprez C, Peno-Mazzarino L, Lati E, Rousseaud A, Declercq L, Kerdine-Römer S. Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation. Antioxidants. 2023; 12(3):766. https://doi.org/10.3390/antiox12030766
Chicago/Turabian StyleSalman, Sara, Cyprien Guermonprez, Laurent Peno-Mazzarino, Elian Lati, Audrey Rousseaud, Lieve Declercq, and Saadia Kerdine-Römer. 2023. "Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation" Antioxidants 12, no. 3: 766. https://doi.org/10.3390/antiox12030766
APA StyleSalman, S., Guermonprez, C., Peno-Mazzarino, L., Lati, E., Rousseaud, A., Declercq, L., & Kerdine-Römer, S. (2023). Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation. Antioxidants, 12(3), 766. https://doi.org/10.3390/antiox12030766