Global Oxidative Status Is Linked to Calcific Aortic Stenosis: The Differences Due to Diabetes Mellitus and the Effects of Metformin
Abstract
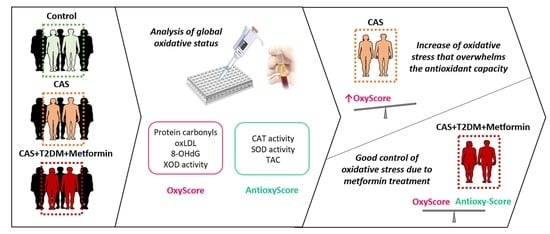
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biomarkers of Oxidative Damage
2.3. Biomarkers of Antioxidant Defense
2.4. OxyScore and AntioxyScore
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Markers of Oxidative Damage
3.3. Markers of Antioxidant Defense
3.4. Global Oxidative Status
3.5. Metformin and Markers of Oxidative Stress and Inflammation
4. Discussion
4.1. Oxidative Status in CAS Patients
4.2. Oxidative Status in CAS Patients with T2DM
4.3. Metformin, Oxidative Stress, and Inflammation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zebhi, B.; Lazkani, M.; Bark, D.J. Calcific Aortic Stenosis—A Review on Acquired Mechanisms of the Disease and Treatments. Front. Cardiovasc. Med. 2021, 8, 734175. [Google Scholar] [CrossRef]
- Freeman, R.V.; Otto, C.M. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation 2005, 111, 3316–3326. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Kamalesh, M.; Ng, C.; El Masry, H.; Eckert, G.; Sawada, S. Does diabetes accelerate progression of calcific aortic stenosis? Eur. J. Echocardiogr. 2009, 10, 723–725. [Google Scholar] [CrossRef]
- Rudolph, T.K.; Messika-Zeitoun, D.; Frey, N.; Thambyrajah, J.; Serra, A.; Schulz, E.; Maly, J.; Aiello, M.; Lloyd, G.; Bortone, A.S.; et al. Impact of selected comorbidities on the presentation and management of aortic stenosis. Open Heart 2020, 7, e001271. [Google Scholar] [CrossRef]
- Nowakowska, M.; Zghebi, S.S.; Ashcroft, D.M.; Buchan, I.; Chew-Graham, C.; Holt, T.; Mallen, C.; Van Marwijk, H.; Peek, N.; Perera-Salazar, R.; et al. The comorbidity burden of type 2 diabetes mellitus: Patterns, clusters and predictions from a large English primary care cohort. BMC Med. 2019, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, H.Z.E.; Zhao, G.; Shah, A.M.; Zhang, M. Role of oxidative stress in calcific aortic valve disease and its therapeutic implications. Cardiovasc. Res. 2021, 118, 1433–1451. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef]
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Dev. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Lamanna, C.; Monami, M.; Marchionni, N.; Mannucci, E. Effect of metformin on cardiovascular events and mortality: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2011, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom Prospective Diabetes Study Group. 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ Br. Med. J. 1995, 310, 83–88. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Morrison, V.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.; Balfour, D.J.; Savinko, T.; Wong, A.K.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.B.A.; Gomes, M.B. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 6–15. [Google Scholar] [CrossRef]
- Rena, G.; Lang, C.C. Repurposing Metformin for Cardiovascular Disease. Circulation 2018, 137, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhu, L.; Liu, J.; Zhu, T.; Xie, Z.; Sun, X.; Zhang, H. Metformin Protects against Oxidative Stress Injury Induced by Ischemia/Reperfusion via Regulation of the lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxid. Med. Cell. Longev. 2019, 2019, 8768327. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Shao, Y.; Wu, C.; Ma, X.; Lv, C.; Wang, Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell. Endocrinol. 2020, 500, 110628. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free. Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Saleh, L.; Plieth, C. Total low-molecular-weight antioxidants as a summary parameter, quantified in biological samples by a chemiluminescence inhibition assay. Nat. Protoc. 2010, 5, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, E.; Navarro-García, J.A.; Ripoll, J.A.; González-Lafuente, L.; Alonso, N.C.; Martinez, P.; Calvo-Bonacho, E.; Alvarez-Llamas, G.; Barderas, M.G.; Ruilope, L.M.; et al. Lifetime cardiovascular risk is associated with a multimarker score of systemic oxidative status in young adults independently of traditional risk factors. Transl. Res. 2019, 212, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, J.A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Abarca-Zabalía, J.; Susmozas-Sánchez, A.; Lafuente, L.G.; Bada-Bosch, T.; Hernández, E.; Mérida-Herrero, E.; Praga, M.; et al. Oxidative Status before and after Renal Replacement Therapy: Differences between Conventional High Flux Hemodialysis and on-Line Hemodiafiltration. Nutrients 2019, 11, 2809. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sánchez, E.; Navarro-García, J.A.; Aceves-Ripoll, J.; González-Lafuente, L.; Corbacho-Alonso, N.; Baldan-Martín, M.; Madruga, F.; Alvarez-Llamas, G.; Barderas, M.G.; Ruilope, L.M.; et al. Analysis of Global Oxidative Status Using Multimarker Scores Reveals a Specific Association Between Renal Dysfunction and Diuretic Therapy in Older Adults. J. Gerontol. Ser. A 2021, 76, 1198–1205. [Google Scholar] [CrossRef]
- Corbacho-Alonso, N.; Baldán-Martín, M.; López, J.A.; Rodríguez-Sánchez, E.; Martínez, P.J.; Mourino-Alvarez, L.; Sastre-Oliva, T.; Cabrera, M.; Calvo, E.; Padial, L.R.; et al. Cardiovascular Risk Stratification Based on Oxidative Stress for Early Detection of Pathology. Antioxid. Redox Signal. 2021, 35, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Oliva, T.; Corbacho-Alonso, N.; Albo-Escalona, D.; Lopez, J.A.; Lopez-Almodovar, L.F.; Vázquez, J.; Padial, L.R.; Mourino-Alvarez, L.; Barderas, M.G. The Influence of Coronary Artery Disease in the Development of Aortic Stenosis and the Importance of the Albumin Redox State. Antioxidants 2022, 11, 317. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Manduteanu, I.; Simionescu, D.; Simionescu, A.; Simionescu, M. Aortic valve disease in diabetes: Molecular mechanisms and novel therapies. J. Cell. Mol. Med. 2021, 25, 9483–9495. [Google Scholar] [CrossRef]
- Adhikari, R.; Shiwakoti, S.; Ko, J.-Y.; Dhakal, B.; Park, S.-H.; Choi, I.J.; Kim, H.J.; Oak, M.-H. Oxidative Stress in Calcific Aortic Valve Stenosis: Protective Role of Natural Antioxidants. Antioxidants 2022, 11, 1169. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Krata, N.; Zagożdżon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Et Ther. Exp. 2018, 66, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age 1997, 20, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Siudut, J.; Natorska, J.; Wypasek, E.; Wiewiórka, Ł.; Ostrowska-Kaim, E.; Wiśniowska-Śmiałek, S.; Plens, K.; Legutko, J.; Undas, A. Impaired Fibrinolysis in Patients with Isolated Aortic Stenosis is Associated with Enhanced Oxidative Stress. J. Clin. Med. 2020, 9, 2002. [Google Scholar] [CrossRef]
- Gwinner, W.; Scheuer, H.; Haller, H.; Brandes, R.; Groene, H.-J. Pivotal role of xanthine oxidase in the initiation of tubulointerstitial renal injury in rats with hyperlipidemia. Kidney Int. 2006, 69, 481–487. [Google Scholar] [CrossRef]
- Kushiyama, A.; Okubo, H.; Sakoda, H.; Kikuchi, T.; Fujishiro, M.; Sato, H.; Kushiyama, S.; Iwashita, M.; Nishimura, F.; Fukushima, T.; et al. Xanthine Oxidoreductase Is Involved in Macrophage Foam Cell Formation and Atherosclerosis Development. Arter. Thromb. Vasc. Biol. 2012, 32, 291–298. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association Between Circulating Oxidized LDL and Atherosclerotic Cardiovascular Disease: A Meta-analysis of Observational Studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid Oxidation Products Have Opposite Effects on Calcifying Vascular Cell and Bone Cell Differentiation. Arter. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef]
- Olsson, M.; Thyberg, J.; Nilsson, J. Presence of Oxidized Low Density Lipoprotein in Nonrheumatic Stenotic Aortic Valves. Arter. Thromb. Vasc. Biol. 1999, 19, 1218–1222. [Google Scholar] [CrossRef]
- Daiber, A.; Hahad, O.; Andreadou, I.; Steven, S.; Daub, S.; Münzel, T. Redox-related biomarkers in human cardiovascular disease—Classical footprints and beyond. Redox Biol. 2021, 42, 101875. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Nyúl-Tóth, Á.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019, 41, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Bigagli, E.; Lodovici, M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxidative Med. Cell. Longev. 2019, 2019, 5953685. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Cammisotto, V.; Nocella, C.; Bartimoccia, S.; Sanguigni, V.; Francomano, D.; Sciarretta, S.; Pastori, D.; Peruzzi, M.; Cavarretta, E.; D’amico, A.; et al. The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors. Antioxidants 2021, 10, 146. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Khan, M.; Paintlia, M.K.; Hoda, M.N.; Giri, S. Metformin Attenuated the Autoimmune Disease of the Central Nervous System in Animal Models of Multiple Sclerosis. J. Immunol. 2009, 182, 8005–8014. [Google Scholar] [CrossRef]
- Ullah, I.; Ullah, N.; Naseer, M.I.; Lee, H.Y.; Kim, M.O. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci. 2012, 13, 11. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Al-Azzam, S.I.; Tashtoush, M.H.; Mhaidat, N.M. Metformin Eased Cognitive Impairment Induced by Chronic L-methionine Administration: Potential Role of Oxidative Stress. Curr. Neuropharmacol. 2014, 12, 186–192. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- Brunmair, B.; Staniek, K.; Gras, F.; Scharf, N.; Althaym, A.; Clara, R.; Roden, M.; Gnaiger, E.; Nohl, H.; Waldhäusl, W.; et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: A common mechanism contributing to their antidiabetic actions? Diabetes 2004, 53, 1052–1059. [Google Scholar] [CrossRef]
- Turrens, J.F.; Boveris, A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980, 191, 421–427. [Google Scholar] [CrossRef]
- Algire, C.; Moiseeva, O.; Deschênes-Simard, X.; Amrein, L.; Petruccelli, L.; Birman, E.; Viollet, B.; Ferbeyre, G.; Pollak, M.N. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev. Res. 2012, 5, 536–543. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chowdhury, S.; Bhattacharyya, M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2011, 93, 56–62. [Google Scholar] [CrossRef]
- Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M.; Wroński, S.; Tarasiuk, J.; Maredziak, M. Metformin Decreases Reactive Oxygen Species, Enhances Osteogenic Properties of Adipose-Derived Multipotent Mesenchymal Stem Cells In Vitro, and Increases Bone Density In Vivo. Oxid. Med. Cell. Longev. 2016, 2016, 9785890. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yang, Q.; Xu, C.; Zheng, Y.; Wang, L.; Wu, J.; Zeng, M.; Luo, M. Metformin prevents methylglyoxal-induced apoptosis by suppressing oxidative stress in vitro and in vivo. Cell Death Dis. 2022, 13, 29. [Google Scholar] [CrossRef]
- Accattato, F.; Greco, M.; Pullano, S.A.; Carè, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Gulletta, E. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- En, Q.; Zeping, H.; Yuetang, W.; Xu, W.; Wei, W. Metformin alleviates the calcification of aortic valve interstitial cells through activating the PI3K/AKT pathway in an AMPK dependent way. Mol. Med. 2021, 27, 156. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]





| Control (n = 20) | CAS (n = 18) | CAS + T2DM + Metformin (n = 18) | p-Value | |
|---|---|---|---|---|
| Age (years) | 71 ± 9 | 76 ± 6 | 72 ± 9 | 0.21 |
| Sex (male) (%) | 50 | 50 | 50 | 1.00 |
| Obesity (%) | 25 | 17 | 28 | 0.71 |
| Hypertension (%) | 90 | 72 | 89 | 0.26 |
| Dyslipidemia (%) | 45 | 67 | 50 | 0.38 |
| Smoker (%) | 10 | 28 | 11 | 0.26 |
| Cholesterol (mg/dL) | 168 ± 46 | 164 ± 24 | 137 ± 36 # | 0.03 |
| HDL (mg/dL) | 55 ± 15 | 53 ± 18 | 39 ± 9 #,†† | 0.00 |
| LDL (mg/dL) | 91 ± 36 | 92 ± 19 | 76 ± 31 | 0.20 |
| Triglycerides (mg/dL) | 106 ± 41 | 94 ± 33 | 110 ± 49 | 0.44 |
| Glycemia (mg/dL) | 96 ± 9 | 100 ± 11 | 139 ± 43 ###,††† | 0.00 |
| Leukocytes (×109/mL) | 7.0 ± 2 | 8.0 ± 2 | 7.7 ± 2 | 0.30 |
| Fibrinogen (mg/dL) | 441 ± 99 | 480 ± 122 | 0.34 | |
| AV area (cm2) | - | 0.82 ± 0.14 | 0.85 ± 0.16 | 0.60 |
| Ejection fraction (%) | - | 59 ± 13 | 57 ± 13 | 0.61 |
| Mean gradient (mmHg) | - | 41 ± 16 | 39 ± 17 | 0.69 |
| Peak gradient (mmHg) | - | 66 ± 25 | 74 ± 28 | 0.45 |
| Systolic diameter (mm) | - | 27 ± 4 | 30 ± 6 | 0.12 |
| Diastolic diameter (mm) | - | 44 ± 6 | 45 ± 6 | 0.55 |
| Septum (mm) | - | 14 ± 2 | 14 ± 2 | 0.47 |
| Drugs | ||||
| Anti-hypertensives (%) | 85 | 83 | 94 | 0.55 |
| Lipid-lowering agents (%) | 65 | 56 | 61 | 0.84 |
| Antidiabetic agent (%) (metformin) | 0 | 0 | 100 | 0.00 |
| r | p-Value | |
|---|---|---|
| Cholesterol * | −0.353 | 0.035 |
| HDL ** | −0.573 | 0.000 |
| LDL | −0.225 | 0.118 |
| Triglycerides | −0.088 | 0.609 |
| r | p-Value | |
|---|---|---|
| Protein carbonyls * | −0.279 | 0.036 |
| oxLDL ** | −0.348 | 0.009 |
| 8-OHdG | −0.044 | 0.746 |
| XOD activity | −0.138 | 0.308 |
| Catalase activity | −0.062 | 0.652 |
| SOD activity | −0.023 | 0.866 |
| TAC | −0.174 | 0.199 |
| OxyScore * | −0.306 | 0.022 |
| AntioxyScore | −0.153 | 0.261 |
| r | p-Value | |
|---|---|---|
| Protein carbonyls | 0.083 | 0.548 |
| oxLDL | 0.104 | 0.454 |
| XOD activity | 0.103 | 0.455 |
| 8-OHdG | 0.079 | 0.566 |
| Catalase activity * | 0.278 | 0.040 |
| SOD activity | −0.116 | 0.399 |
| TAC ** | −0.363 | 0.006 |
| OxyScore | 0.185 | 0.177 |
| AntioxyScore | −0.102 | 0.457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corbacho-Alonso, N.; Rodríguez-Sánchez, E.; Sastre-Oliva, T.; Mercado-García, E.; Perales-Sánchez, I.; Juarez-Alia, C.; López-Almodovar, L.F.; Padial, L.R.; Tejerina, T.; Mourino-Alvarez, L.; et al. Global Oxidative Status Is Linked to Calcific Aortic Stenosis: The Differences Due to Diabetes Mellitus and the Effects of Metformin. Antioxidants 2023, 12, 1024. https://doi.org/10.3390/antiox12051024
Corbacho-Alonso N, Rodríguez-Sánchez E, Sastre-Oliva T, Mercado-García E, Perales-Sánchez I, Juarez-Alia C, López-Almodovar LF, Padial LR, Tejerina T, Mourino-Alvarez L, et al. Global Oxidative Status Is Linked to Calcific Aortic Stenosis: The Differences Due to Diabetes Mellitus and the Effects of Metformin. Antioxidants. 2023; 12(5):1024. https://doi.org/10.3390/antiox12051024
Chicago/Turabian StyleCorbacho-Alonso, Nerea, Elena Rodríguez-Sánchez, Tamara Sastre-Oliva, Elisa Mercado-García, Ines Perales-Sánchez, Cristina Juarez-Alia, Luis F. López-Almodovar, Luis R. Padial, Teresa Tejerina, Laura Mourino-Alvarez, and et al. 2023. "Global Oxidative Status Is Linked to Calcific Aortic Stenosis: The Differences Due to Diabetes Mellitus and the Effects of Metformin" Antioxidants 12, no. 5: 1024. https://doi.org/10.3390/antiox12051024
APA StyleCorbacho-Alonso, N., Rodríguez-Sánchez, E., Sastre-Oliva, T., Mercado-García, E., Perales-Sánchez, I., Juarez-Alia, C., López-Almodovar, L. F., Padial, L. R., Tejerina, T., Mourino-Alvarez, L., Ruiz-Hurtado, G., & Barderas, M. G. (2023). Global Oxidative Status Is Linked to Calcific Aortic Stenosis: The Differences Due to Diabetes Mellitus and the Effects of Metformin. Antioxidants, 12(5), 1024. https://doi.org/10.3390/antiox12051024