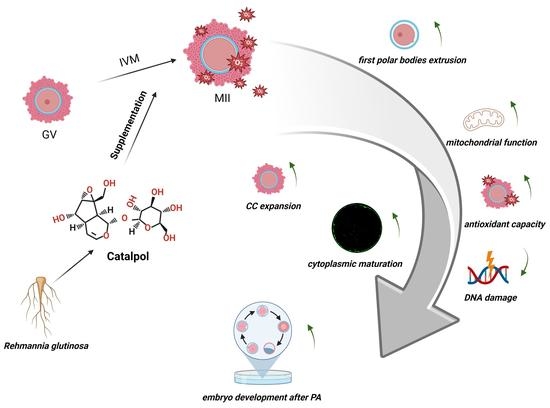
Beneficial Effects of Catalpol Supplementation during In Vitro Maturation of Porcine Cumulus-Oocyte Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. IVM of Porcine COCs
2.3. PA of Porcine Oocytes
2.4. cDNA Acquisition from MII Stage Porcine Oocytes
2.5. RNA Isolation and Real-Time Quantitative PCR
2.6. Evaluation of CC Expansion
2.7. Examination of Mitochondrial Membrane Potential in Oocytes
2.8. Examination of Mitochondrial Distribution in Oocytes
2.9. ROS Assay
2.10. Total GSH Determination
2.11. Malondialdehyde (MDA) Assay
2.12. Detection of CG Distribution in Porcine Oocytes
2.13. Immunofluorescence Staining
2.14. Blastocyst Hoechst33342 Staining
2.15. Statistical Analysis
3. Results
3.1. Effects of Catalpol on Extrusion of the First Polar Body
3.2. Effects of Catalpol on CC Expansion in Porcine COCs
3.3. Effects of Catalpol Supplementation on the Distribution of CGs inPorcine MII Stage Oocytes
3.4. Effects of Catalpol on Mitochondrial Activity and Function in Porcine MII Stage Oocytes
3.5. Effects of Catalpol on Antioxidant Capacity in Porcine MII Stage Oocytes
3.6. Effects of Catalpol on DNA Damage in Porcine MII Stage Oocytes
3.7. Effects of Catalpol on Embryonic Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almubarak, A.M.; Kim, E.; Yu, I.J.; Jeon, Y. Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 2021, 169, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Anim. Int. J. Anim. Biosci. 2020, 14, 991–1004. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. RBE 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells 2022, 11, 3573. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Jin, W.; Kam, M.K.; Lee, S.W.; Park, Y.H.; Lee, H.J.; Lee, D.S. Peroxiredoxin 2 Ameliorates AβO-Mediated Autophagy by Inhibiting ROS via the ROS-NRF2-p62 Pathway in N2a-APP Swedish Cells. Antioxidants 2022, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postep. Hig. I Med. Dosw. 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; Cackler, A.B.; Arnett, M.G. Why should you care about free radicals? RN 2004, 67, 38–42. [Google Scholar]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Kala, M.; Shaikh, M.V.; Nivsarkar, M. Equilibrium between anti-oxidants and reactive oxygen species: A requisite for oocyte development and maturation. Reprod. Med. Biol. 2017, 16, 28–35. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Morado, S.A.; Cetica, P.D.; Beconi, M.T.; Dalvit, G.C. Reactive oxygen species in bovine oocyte maturation in vitro. Reprod. Fertil. Dev. 2009, 21, 608–614. [Google Scholar] [CrossRef]
- Paramio, M.T.; Izquierdo, D. Current status of in vitro embryo production in sheep and goats. Reprod. Domest. Anim. Zuchthyg. 2014, 49 (Suppl. 4), 37–48. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jia, R.; Wang, F.; Qiu, G.; Qiao, P.; Xu, X.; Wu, D. Catalpol protects mice against Lipopolysaccharide/D-galactosamine-induced acute liver injury through inhibiting inflammatory and oxidative response. Oncotarget 2018, 9, 3887–3894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.Y.; Zheng, C.Z.; Hao, X.P.; Zhang, D.J.; Mao, A.W.; Yuan, P. Catalpol ameliorates diabetic atherosclerosis in diabetic rabbits. Am. J. Transl. Res. 2016, 8, 4278–4288. [Google Scholar] [PubMed]
- Silva, A.F.B.; Lima, L.F.; Morais, A.N.P.; Lienou, L.L.; Watanabe, Y.F.; Joaquim, D.C.; Morais, S.M.; Alves, D.R.; Pereira, A.F.; Santos, A.C.; et al. Oocyte in vitro maturation with eugenol improves the medium antioxidant capacity and total cell number per blastocyst. Theriogenology 2022, 192, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xuan, B.; Xu, D.; Wang, Q.; Cheng, M.; Jin, Y. Crocin supplementation during oocyte maturation enhances antioxidant defence and subsequent cleavage rate. Reprod. Domest. Anim. Zuchthyg. 2019, 54, 300–308. [Google Scholar] [CrossRef]
- Xiang, D.C.; Jia, B.Y.; Fu, X.W.; Guo, J.X.; Hong, Q.H.; Quan, G.B.; Wu, G.Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef]
- You, L.; Peng, H.; Liu, J.; Cai, M.; Wu, H.; Zhang, Z.; Bai, J.; Yao, Y.; Dong, X.; Yin, X.; et al. Catalpol Protects ARPE-19 Cells against Oxidative Stress via Activation of the Keap1/Nrf2/ARE Pathway. Cells 2021, 10, 2635. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Zhang, M.; Sun, S.; Zhong, Y.; Han, L.; Xu, Y.; Wan, D.; Zhang, J.; Zhu, H. Feasibility of Catalpol Intranasal Administration and Its Protective Effect on Acute Cerebral Ischemia in Rats via Anti-Oxidative and Anti-Apoptotic Mechanisms. Drug Des. Dev. Ther. 2022, 16, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.R.; Jiang, L.; Duan, Y.L.; An, L.J.; Jiang, B. Efficacy of catalpol as protectant against oxidative stress and mitochondrial dysfunction on rotenone-induced toxicity in mice brain. Environ. Toxicol. Pharmacol. 2007, 23, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yamashita, S.; Hoshi, H. Influence of epidermal growth factor and transforming growth factor-alpha on in vitro maturation of cumulus cell-enclosed bovine oocytes in a defined medium. J. Reprod. Fertil. 1994, 100, 439–446. [Google Scholar] [CrossRef]
- Ali, A.A.; Bilodeau, J.F.; Sirard, M.A. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 2003, 59, 939–949. [Google Scholar] [CrossRef]
- Kere, M.; Siriboon, C.; Lo, N.W.; Nguyen, N.T.; Ju, J.C. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J. Reprod. Dev. 2013, 59, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, J.; Jiang, B.; Liu, J.H.; Lei, C.; Zhang, X.L.; An, L.J. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci. Lett. 2008, 442, 224–227. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.; Yang, X.; Liu, X.; Deng, K.; Chen, M.; Yan, X.; Lu, F.; Shi, D. Beneficial effects of fibroblast growth factor 10 supplementation during in vitro maturation of buffalo cumulus-oocyte complexes. Theriogenology 2023, 201, 126–137. [Google Scholar] [CrossRef]
- Rose, R.D.; Gilchrist, R.B.; Kelly, J.M.; Thompson, J.G.; Sutton-McDowall, M.L. Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology 2013, 79, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Dong, Y.; Zhang, Y.; Wu, X.; Zhang, X.; Liang, Y.; Li, Y.; Zeng, F.; Shi, J.; Zhou, R.; et al. Supplementation of SDF1 during Pig Oocyte In Vitro Maturation Improves Subsequent Embryo Development. Molecules 2022, 27, 6830. [Google Scholar] [CrossRef]
- Senbon, S.; Hirao, Y.; Miyano, T. Interactions between the oocyte and surrounding somatic cells in follicular development: Lessons from in vitro culture. J. Reprod. Dev. 2003, 49, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Wongsrikeao, P.; Kaneshige, Y.; Ooki, R.; Taniguchi, M.; Agung, B.; Nii, M.; Otoi, T. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reprod. Domest. Anim. Zuchthyg. 2005, 40, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Maeda, T.; Terada, T. Dynamic changes of connexin-43, gap junctional protein, in outer layers of cumulus cells are regulated by PKC and PI 3-kinase during meiotic resumption in porcine oocytes. Biol. Reprod. 2001, 64, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Eppig, J.J. The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev. Biol. 1982, 89, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Cajas, Y.N.; Cañón-Beltrán, K.; Ladrón de Guevara, M.; Millán de la Blanca, M.G.; Ramos-Ibeas, P.; Gutiérrez-Adán, A.; Rizos, D.; González, E.M. Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality. Int. J. Mol. Sci. 2020, 21, 5340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, H.; Li, Y.; Zhang, Y.; Liang, Y.; Shi, J.; Zhou, R.; Hong, L.; Cai, G.; Wu, Z.; et al. Amphiregulin Supplementation During Pig Oocyte In Vitro Maturation Enhances Subsequent Development of Cloned Embryos by Promoting Cumulus Cell Proliferation. Cell. Reprogram. 2022, 24, 175–185. [Google Scholar] [CrossRef]
- Russell, D.L.; Robker, R.L. Molecular mechanisms of ovulation: Co-ordination through the cumulus complex. Hum. Reprod. Update 2007, 13, 289–312. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Miao, Y.; Zhou, C.; Cui, Z.; Tang, L.; ShiYang, X.; Lu, Y.; Zhang, M.; Dai, X.; Xiong, B. Dynein promotes porcine oocyte meiotic progression by maintaining cytoskeletal structures and cortical granule arrangement. Cell Cycle 2017, 16, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Kulus, M.; Kranc, W.; Jeseta, M.; Sujka-Kordowska, P.; Konwerska, A.; Ciesiółka, S.; Celichowski, P.; Moncrieff, L.; Kocherova, I.; Józkowiak, M.; et al. Cortical Granule Distribution and Expression Pattern of Genes Regulating Cellular Component Size, Morphogenesis, and Potential to Differentiation are Related to Oocyte Developmental Competence and Maturational Capacity In Vivo and In Vitro. Genes 2020, 11, 815. [Google Scholar] [CrossRef]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.; Lopes, F.G.; Leal, C. Effects of supplementation of medium with different antioxidants during in vitro maturation of bovine oocytes on subsequent embryo production. Reprod. Domest. Anim. Zuchthyg. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169 (Suppl. 2), 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Tang, S.; Jiang, Y.; Long, F.; He, F.; Liu, J.; Gu, S.; Lu, Y.; Yin, Z. Oxidative stress induces meiotic defects of oocytes in a mouse psoriasis model. Cell Death Dis. 2022, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Ouyang, Y.C.; Meng, T.G.; Zhang, H.Y.; Yue, W.; Yan, F.Z.; Xue, Y.; Schatten, H.; Wang, Z.B.; Sun, Q.Y. OTSSP167 leads to follicular dysplasia and negatively affects oocyte quality in mice. Toxicology 2022, 476, 153243. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Jin, Y.; Hao, J.; Huang, S.; Wang, D.; Quan, F.; Ren, W.; Zhang, J.; Zhang, M.; Yu, X. Procyanidin B1 promotes in vitro maturation of pig oocytes by reducing oxidative stress. Mol. Reprod. Dev. 2021, 88, 55–66. [Google Scholar] [CrossRef]
- Nie, J.; Yan, K.; Sui, L.; Zhang, H.; Zhang, H.; Yang, X.; Lu, S.; Lu, K.; Liang, X. Mogroside V improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology 2020, 141, 35–40. [Google Scholar] [CrossRef]
- Mun, S.E.; Sim, B.W.; Yoon, S.B.; Jeong, P.S.; Yang, H.J.; Choi, S.A.; Park, Y.H.; Kim, Y.H.; Kang, P.; Jeong, K.J.; et al. Dual effect of fetal bovine serum on early development depends on stage-specific reactive oxygen species demands in pigs. PLoS ONE 2017, 12, e0175427. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Park, S.Y.; Kim, J.W.; Yang, S.G.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Choo, Y.K.; Koo, D.B. Melatonin Improves Oocyte Maturation and Mitochondrial Functions by Reducing Bisphenol A-Derived Superoxide in Porcine Oocytes In Vitro. Int. J. Mol. Sci. 2018, 19, 3422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Matos, D.G.; Furnus, C.C.; Moses, D.F. Glutathione synthesis during in vitro maturation of bovine oocytes: Role of cumulus cells. Biol. Reprod. 1997, 57, 1420–1425. [Google Scholar] [CrossRef] [Green Version]
- Luberda, Z. The role of glutathione in mammalian gametes. Reprod. Biol. 2005, 5, 5–17. [Google Scholar]
- Li, Y.; Zhang, Z.; He, C.; Zhu, K.; Xu, Z.; Ma, T.; Tao, J.; Liu, G. Melatonin protects porcine oocyte in vitro maturation from heat stress. J. Pineal Res. 2015, 59, 365–375. [Google Scholar] [CrossRef]
- Combelles, C.M.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef] [PubMed]
- Fakruzzaman, M.; Ghanem, N.; Bang, J.I.; Ha, A.N.; Lee, K.L.; Sohn, S.H.; Wang, Z.; Lee, D.S.; Kong, I.K. Effect of peroxiredoxin II on the quality and mitochondrial activity of pre-implantation bovine embryos. Anim. Reprod. Sci. 2015, 159, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhao, S.; Sun, Y.; Jiang, X.; Hao, H.; Du, W.; Zhu, H. Protective effects of melatonin on the in vitro developmental competence of bovine oocytes. Anim. Sci. J. Nihon Chikusan Gakkaiho 2018, 89, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Zhang, Y.; ShiYang, X.; Zhang, Y.; Li, Y.; Shi, X.; Xiong, B. Exposure to aristolochic acid I compromises the maturational competency of porcine oocytes via oxidative stress-induced DNA damage. Aging 2019, 11, 2241–2252. [Google Scholar] [CrossRef]






| Genes | Primer Sequences | Length (bp) | Tm (°C) | Accession No. |
|---|---|---|---|---|
| β-actin | F: GATGACGATATTGCTGCGCTjayjayR: TTCTCCATGTCGTCCCAGTT | 248 | 60 | XM_021086047.1 |
| HAS2 | F: GAGTTTCTCTCCTCCTTGGAAjayjayR: ATGGGGGTTTCTAGAGATTTT | 181 | 60 | NM_214053.1 |
| PTX3 | F: GCTCTCTGGTCTGCAGTGTTGjayjayR: CGCATCTGGGAGTTCTCTAGC | 179 | 60 | NM_001244783.1 |
| PTGS2 | F: ATTTGTTGAATCATTTAGCjayjayR: CATTTCCTTCTCTCCTGTA | 199 | 60 | NM_214321.1 |
| SOD1 | F: GGGCACCATCTACTTCGAGCTjayjayR: CTCTCTTGATCCTTTGGCCCA | 189 | 60 | NM_001190422.1 |
| CAT | F: AGATGAAGCATTGGAAGGAGC jayjayR: TCTCAGGAATTCTCTCCCGGT | 183 | NM_214301.2 | |
| GPX1 | F: ACTACACCCAGATGAATGAGCjayjayR: ACACTTCTCGAAGAGCATGAA | 182 | 60 | NM_214407.1 |
| Catalpol Concentrations (µmol/L) | No. of Oocytes | Replicates | Rate of PB1 (%) |
|---|---|---|---|
| 0 | 150 | 3 | 61.07 ± 2.80 c |
| 5 | 155 | 3 | 65.20 ± 1.82 ab |
| 10 | 145 | 3 | 72.33 ± 1.93 a |
| 50 | 149 | 3 | 66.93 ± 3.52 ab |
| 100 | 146 | 3 | 62.27 ± 2.11 bc |
| Catalpol Concentrations (µmol/L) | No. of Embryos | No. of Cleavage (%) | No. of Blastocyst (%) |
|---|---|---|---|
| 0 | 150 | 80 (52.67 ± 1.16 c) | 21 (13.99 ± 1.81 c) |
| 5 | 155 | 87 (56.15 ± 1.60 bc) | 26 (16.73 ± 0.97 c) |
| 10 | 145 | 96 (66.14 ± 2.80 a) | 37 (25.45 ± 1.81 a) |
| 50 | 149 | 88 (58.97 ± 2.13 b) | 29 (19.44 ± 1.38 b) |
| 100 | 146 | 78 (53.40 ± 1.52 bc) | 24 (16.40 ± 1.22 c) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, Y.; Li, S.; Yan, X.; Yang, X.; Chen, M.; Wang, Y.; Jia, R.; Zhou, D.; Shi, D.; et al. Beneficial Effects of Catalpol Supplementation during In Vitro Maturation of Porcine Cumulus-Oocyte Complexes. Antioxidants 2023, 12, 1222. https://doi.org/10.3390/antiox12061222
Wang Y, Xu Y, Li S, Yan X, Yang X, Chen M, Wang Y, Jia R, Zhou D, Shi D, et al. Beneficial Effects of Catalpol Supplementation during In Vitro Maturation of Porcine Cumulus-Oocyte Complexes. Antioxidants. 2023; 12(6):1222. https://doi.org/10.3390/antiox12061222
Chicago/Turabian StyleWang, Yanxin, Ye Xu, Sijia Li, Xi Yan, Xiaofen Yang, Mengjia Chen, Yun Wang, Ruru Jia, Dongping Zhou, Deshun Shi, and et al. 2023. "Beneficial Effects of Catalpol Supplementation during In Vitro Maturation of Porcine Cumulus-Oocyte Complexes" Antioxidants 12, no. 6: 1222. https://doi.org/10.3390/antiox12061222
APA StyleWang, Y., Xu, Y., Li, S., Yan, X., Yang, X., Chen, M., Wang, Y., Jia, R., Zhou, D., Shi, D., & Lu, F. (2023). Beneficial Effects of Catalpol Supplementation during In Vitro Maturation of Porcine Cumulus-Oocyte Complexes. Antioxidants, 12(6), 1222. https://doi.org/10.3390/antiox12061222