Association of Inflammatory and Oxidative Status Markers with Metabolic Syndrome and Its Components in 40-to-45-Year-Old Females: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measurements
2.3. Calculations and Classification
2.4. Statistical Analysis
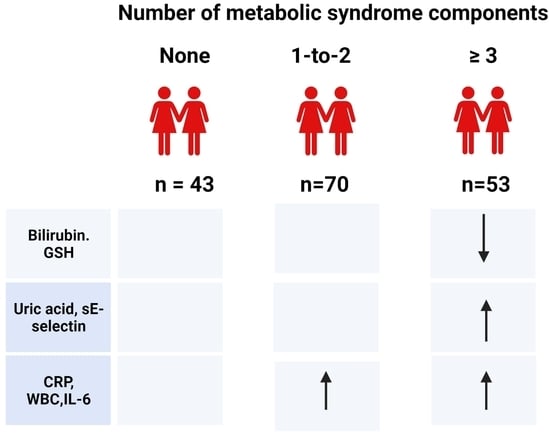
3. Results
3.1. General Characteristics
3.2. Markers of Oxidative Status
3.2.1. Plasma Levels of Lipoxidation and Glycoxidation Products
3.2.2. Endogenous Antioxidants
3.2.3. Exogenous Antioxidants
3.2.4. Antioxidants of Mixed Origin
3.3. Markers of Inflammatory Status
3.3.1. Pro- and Anti-Inflammatory Markers
3.3.2. Soluble Adhesion Molecules
3.3.3. Soluble Receptor for Advanced Glycation End Products
3.4. Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Balkau, B.; Charles, M.A. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet. Med. 1999, 16, 442–443. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program Expert Panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Magliano, D.; Matsuzawa, Y.; Alberti, G.; Shaw, J. The metabolic syndrome: A global public health problem and a new definition. J. Atheroscler. Thromb. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Ford, E.S.; Li, C.; Sattar, N. Metabolic syndrome and incident diabetes: Current state of the evidence. Diabetes Care 2008, 31, 1898–1904. [Google Scholar] [CrossRef] [Green Version]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk a Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [Green Version]
- Spahis, S.; Borys, J.M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [Green Version]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Rezzani, R.; Franco, C. Liver, Oxidative Stress and Metabolic Syndromes. Nutrients 2021, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliucci, A. Biomarkers of dysfunctional visceral fat. Adv. Clin. Chem. 2022, 109, 1–30. [Google Scholar]
- Hachiya, R.; Tanaka, M.; Itoh, M.; Suganami, T. Molecular mechanism of cross-talk between immune and metabolic systems in metabolic syndrome. Inflamm. Regen. 2022, 42, 13. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell. Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Henning, R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: A review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504–529. [Google Scholar]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef]
- Artemniak-Wojtowicz, D.; Kucharska, A.M.; Pyrżak, B. Obesity and chronic inflammation crosslinking. Cent. Eur. J. Immunol. 2020, 45, 461–468. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils Actively Contribute to Obesity-Associated Inflammation and Pathological Complications. Cells 2022, 11, 1883. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Pradhan, A.D.; Ridker, P.M. Interrelationships between inflammation, C-reactive protein, and insulin resistance. J. Cardiometab. Syndr. 2006, 1, 190–196. [Google Scholar] [CrossRef]
- Yasue, H.; Hirai, N.; Mizuno, Y.; Harada, E.; Itoh, T.; Yoshimura, M.; Kugiyama, K.; Ogawa, H. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ. J. 2006, 70, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Verhaegen, D.; Smits, K.; Osório, N.; Caseiro, A. Oxidative Stress in Relation to Aging and Exercise. Encyclopedia 2022, 2, 1545–1558. [Google Scholar] [CrossRef]
- Münzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef] [Green Version]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2022, 11, 79. [Google Scholar] [CrossRef]
- Suriyaprom, K.; Kaewprasert, S.; Putpadungwipon, P.; Namjuntra, P.; Klongthalay, S. Association of antioxidant status and inflammatory markers with metabolic syndrome in Thais. J. Health Popul. Nutr. 2019, 38, 1. [Google Scholar] [CrossRef] [Green Version]
- Zurawska-Plaksej, E.; Grzebyk, E.; Marciniak, D.; Szymanska-Chabowska, A.; Piwowar, A. Oxidatively modified forms of albumin in patients with risk factors of metabolic syndrome. J. Endocrinol. Invest. 2014, 37, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Tylutka, A.; Morawin, B.; Walas, Ł.; Michałek, M.; Gwara, A.; Zembron-Lacny, A. Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci. Rep. 2023, 13, 89. [Google Scholar] [CrossRef]
- You, T.; Nicklas, B.J.; Ding, J.; Penninx, B.W.; Goodpaster, B.H.; Bauer, D.C.; Tylavsky, F.A.; Harris, T.B.; Kritchevsky, S.B. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-J.; Yen, C.-H.; Huang, Y.-C.; Lee, B.-J.; Hsia, S.; Lin, P.-T. Relationships between Inflammation, Adiponectin, and Oxidative Stress in Metabolic Syndrome. PLoS ONE 2012, 7, e45693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirhafez, S.R.; Pasdar, A.; Avan, A.; Esmaily, H.; Moezzi, A.; Mohebati, M.; Meshkat, Z.; Mehrad-Majd, H.; Eslami, S.; Rahimi, H.R.; et al. Cytokine and growth factor profiling in patients with the metabolic syndrome. Br. J. Nutr. 2015, 113, 1911–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christiana, U.I.; Casimir, A.E.; Nicholas, A.A.; Christian, M.C.; Obiefuna, A.I. Plasma levels of inflammatory cytokines in adult Nigerians with the metabolic syndrome. Niger. Med. J. 2016, 57, 64–68. [Google Scholar] [CrossRef]
- González-Jiménez, E.; Schmidt-RioValle, J.; Montero-Alonso, M.A.; Padez, C.; García-García, C.J.; Perona, J.S. Influence of Biochemical and Anthropometric Factors on the Presence of Insulin Resistance in Adolescents. Biol. Res. Nurs. 2016, 18, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.K.; Ford, E.S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am. J. Med. 2007, 120, 442–447. [Google Scholar] [CrossRef]
- Neves, C.V.B.; Mambrini, J.V.M.; Torres, K.C.L.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Lima-Costa, M.F.; Peixoto, S.V. Association of metabolic syndrome with inflammatory markers in a sample of community-dwelling older adults. Cad. Saude Publica 2019, 35, e00129918. [Google Scholar]
- Vaidya, D.; Szklo, M.; Cushman, M.; Holvoet, P.; Polak, J.; Bahrami, H.; Jenny, N.S.; Ouyang, P. Association of endothelial and oxidative stress with metabolic syndrome and subclinical atherosclerosis: Multi-ethnic study of atherosclerosis. Eur. J. Clin. Nutr. 2011, 65, 818–825. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Gonzalez-Gil, A.M.; Villarreal-Calderón, J.R.; Tamez-Rivera, O.; Rodriguez-Gutierrez, N.A.; Castillo, E.C.; Silva-Platas, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Serum Irisin Levels, Endothelial Dysfunction, and Inflammation in Pediatric Patients with Type 2 Diabetes Mellitus and Metabolic Syndrome. J. Diabetes Res. 2020, 2020, 1949415. [Google Scholar] [CrossRef]
- Lee, C.H.; Kuo, F.C.; Tang, W.H.; Lu, C.H.; Su, S.C.; Liu, J.S.; Hsieh, C.H.; Hung, Y.J.; Lin, F.H. Serum E-selectin concentration is associated with risk of metabolic syndrome in females. PLoS ONE 2019, 14, e0222815. [Google Scholar] [CrossRef] [Green Version]
- Ghazizadeh, H.; Rezaei, M.; Avan, A.; Fazilati, M.; Pasdar, A.; Tavallaie, S.; Kazemi, E.; Seyedi, S.M.R.; Ferns, G.A.; Azimi-Nezhad, M.; et al. Association between serum cell adhesion molecules with hs-CRP, uric acid and VEGF genetic polymorphisms in subjects with metabolic syndrome. Mol. Biol. Rep. 2020, 47, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Gurecka, R.; Koborova, I.; Csongova, M.; Sebek, J.; Sebekova, K. Correlation among soluble receptors for advanced glycation end-products, soluble vascular adhesion protein-1/semicarbazide-sensitive amine oxidase (sVAP-1) and cardiometabolic risk markers in apparently healthy adolescents: A cross-sectional study. Glycoconj. J. 2016, 33, 599–606. [Google Scholar] [CrossRef]
- Wu, S.-S.; Kor, C.-T.; Chen, T.-Y.; Liu, K.-H.; Shih, K.-L.; Su, W.-W.; Wu, H.-M. Relationships between Serum Uric Acid, Malondialdehyde Levels, and Carotid Intima-Media Thickness in the Patients with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2019, 2019, 6859757. [Google Scholar] [CrossRef] [PubMed]
- Klisic, A.; Kocic, G.; Kavaric, N.; Pavlovic, R.; Soldatovic, I.; Ninic, A. Nitric Oxide Products are not Associated with Metabolic Syndrome. J. Med. Biochem. 2019, 38, 361–367. [Google Scholar] [CrossRef]
- Venturini, D.; Simao, A.N.C.; Dichi, I. Advanced oxidation protein products are more related to metabolic syndrome components than biomarkers of lipid peroxidation. Nutr. Res. 2015, 35, 759–765. [Google Scholar] [CrossRef]
- Vávrová, L.; Kodydková, J.; Zeman, M.; Dušejovská, M.; Macášek, J.; Staňková, B.; Tvrzická, E.; Zák, A. Altered activities of antioxidant enzymes in patients with metabolic syndrome. Obes. Facts 2013, 6, 39–47. [Google Scholar] [CrossRef]
- Mohorko, N.; Petelin, A.; Jurdana, M.; Biolo, G.; Jenko-Pražnikar, Z. Elevated serum levels of cysteine and tyrosine: Early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. Biomed. Res. Int. 2015, 2015, 418681. [Google Scholar] [CrossRef] [Green Version]
- Awadallah, S.; Hasan, H.; Attlee, A.; Raigangar, V.; Unnikannan, H.; Madkour, M.; Abraham, M.S.; Rashid, L.M. Waist circumference is a major determinant of oxidative stress in subjects with and without metabolic syndrome. Diabetes Metab. Syndr. 2019, 13, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Ziobro, A.; Duchnowicz, P.; Mulik, A.; Koter-Michalak, M.; Broncel, M. Oxidative damages in erythrocytes of patients with metabolic syndrome. Mol. Cell. Biochem. 2013, 378, 267–273. [Google Scholar] [CrossRef] [Green Version]
- González-Jiménez, E.; Schmidt-Riovalle, J.; Sinausía, L.; Carmen Valenza, M.; Perona, J.S. Predictive value of ceruloplasmin for metabolic syndrome in adolescents. Biofactors 2016, 42, 163–170. [Google Scholar]
- Kim, C.H.; Park, J.Y.; Kim, J.Y.; Choi, C.S.; Kim, Y.I.; Chung, Y.E.; Lee, M.S.; Hong, S.K.; Lee, K.U. Elevated serum ceruloplasmin levels in subjects with metabolic syndrome: A population-based study. Metabolism 2002, 51, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.M.; Ziaei, R.; Maracy, M.R. Association of serum ceruloplasmin level with obesity: Some components of metabolic syndrome and high-sensitive C-reactive protein in Iran. J. Obes. 2012, 2012, 951093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebekova, K.; Krivosikova, Z.; Gajdos, M. Total plasma Nepsilon-(carboxymethyl)lysine and sRAGE levels are inversely associated with a number of metabolic syndrome risk factors in non-diabetic young-to-middle-aged medication-free subjects. Clin. Chem. Lab. Med. 2014, 52, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.I.; Dong, C.; Gardener, H.; Elkind, M.S.V.; Wright, C.B.; Goldberg, R.; Sacco, R.L.; Rundek, T. Serum levels of soluble receptor for advanced glycation end-products and metabolic syndrome: The Northern Manhattan Study. Metabolism 2014, 63, 1125–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, M.; Knani, I.; Bouzidi, H.; Berriche, O.; Hammami, M.; Kerkeni, M. Plasma Levels of Pentosidine, Carboxymethyl-Lysine, Soluble Receptor for Advanced Glycation End Products, and Metabolic Syndrome: The Metformin Effect. Dis. Markers 2016, 2016, 6248264. [Google Scholar] [CrossRef] [Green Version]
- He, C.T.; Lee, C.H.; Hsieh, C.H.; Hsiao, F.C. Soluble form of receptor for advanced glycation end products is associated with obesity and metabolic syndrome in adolescents. Int. J. Endocrinol. 2014, 2014, 657607. [Google Scholar] [CrossRef] [Green Version]
- Smolková, B.; Bonassi, S.; Buociková, V.; Dušinská, M.; Horská, A.; Kuba, D.; Džupinková, Z.; Rašlová, K.; Gašparovič, J.; Slíž, I.; et al. Genetic determinants of quantitative traits associated with cardiovascular disease risk. Mutat. Res. 2015, 778, 18–25. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S. A proposal for a primary screening tool: ‘Keep your waist circumference to less than half your height’. BMC Med. 2014, 12, 207. [Google Scholar] [CrossRef] [Green Version]
- Munch, G.; Keis, R.; Wessels, A.; Riederer, P.; Bahner, U.; Heidland, A.; Niwa, T.; Lemke, H.D.; Schinzel, R. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 669–677. [Google Scholar] [CrossRef]
- Yu, P.H.; Zuo, D.M. Oxidative deamination of methylamine by semicarbazide-sensitive amine oxidase leads to cytotoxic damage in endothelial cells. Possible consequences for diabetes. Diabetes 1993, 42, 594–603. [Google Scholar] [CrossRef]
- Richard, M.J.; Guiraud, P.; Meo, J.; Favier, A. High-performance liquid chromatographic separation of malondialdehyde-thiobarbituric acid adduct in biological materials (plasma and human cells) using a commercially available reagent. J. Chromatogr. 1992, 577, 9–18. [Google Scholar] [CrossRef]
- Cerhata, D.; Bauerová, A.; Ginter, E. Determination of ascorbic acid in blood serum using high-performance liquid chromatography and its correlation with spectrophotometric (colorimetric) determination. Ceska Slov. Farm. 1994, 43, 166–168. [Google Scholar] [PubMed]
- Hess, D.; Keller, H.E.; Oberlin, B.; Bonfanti, R.; Schüep, W. Simultaneous determination of retinol, tocopherols, carotenes and lycopene in plasma by means of high-performance liquid chromatography on reversed phase. Int. J. Vitam. Nutr. Res. 1991, 61, 232–238. [Google Scholar] [PubMed]
- Houze, P.; Gamra, S.; Madelaine, I.; Bousquet, B.; Gourmel, B. Simultaneous determination of total plasma glutathione, homocysteine, cysteinylglycine, and methionine by high-performance liquid chromatography with electrochemical detection. J. Clin. Lab. Anal. 2001, 15, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, S.; Pogribna, M.; Pogribny, I.; Hine, R.J.; James, S.J. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J. Nutr. Biochem. 1999, 10, 490–497. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Cavarocchi, N.C.; England, M.D.; O’Brien, J.F.; Solis, E.; Russo, P.; Schaff, H.V.; Orszulak, T.A.; Pluth, J.R.; Kaye, M.P. Superoxide generation during cardiopulmonary bypass: Is there a role for vitamin E? J. Surg. Res. 1986, 40, 519–527. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Schosinsky, K.H.; Lehmann, H.P.; Beeler, M.F. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin. Chem. 1974, 20, 1556–1563. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Shroff, M.R.; Chen, X.; Beydoun, H.A.; Wang, Y.; Zonderman, A.B. Serum antioxidant status is associated with metabolic syndrome among U.S. adults in recent national surveys. J. Nutr. 2011, 141, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Kocna, P. MiniEncyklopedia Laboratorních Metod v Gastroenterologii [MiniEncyclopedia of Laboratory Methods in Gastroenterology]. Available online: http://www1.lf1.cuni.cz/~kocna/glab/glency1.htm (accessed on 13 January 2023).
- Dobiasova, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Bolton, K.; Culleton, B.; Harvey, K.S.; Ikizler, T.A.; Johnson, C.A.; Kausz, A.; Kimmel, P.J.; Kusek, J.; et al. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Sebekova, K.; Stefikova, K.; Polakovicova, D.; Spustova, V.; Dzurik, R. Does magnesium dysbalance participate in the development of insulin resistance in early stages of renal disease? Physiol. Res. 2002, 51, 605–612. [Google Scholar] [PubMed]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Godala, M.; Materek-Kuśmierkiewicz, I.; Moczulski, D.; Rutkowski, M.; Szatko, F.; Gaszyńska, E.; Tokarski, S.; Kowalski, J. The risk of plasma vitamin A, C, E and D deficiency in patients with metabolic syndrome: A case-control study. Adv. Clin. Exp. Med. 2017, 26, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Peña-Orihuela, P.; Perez-Martinez, P.; Fuentes, F.; Marin, C.; Tunez, I.; Tinahones, F.J.; Perez-Jimenez, F.; Roche, H.M.; et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp. Mol. Med. 2013, 45, e28. [Google Scholar] [CrossRef] [Green Version]
- Giral, P.; Jacob, N.; Dourmap, C.; Hansel, B.; Carrié, A.; Bruckert, E.; Girerd, X.; Chapman, M.J. Elevated gamma-glutamyltransferase activity and perturbed thiol profile are associated with features of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [Green Version]
- Vítek, L.; Jirásková, A.; Malíková, I.; Dostálová, G.; Eremiášová, L.; Danzig, V.; Linhart, A.; Haluzík, M. Serum Bilirubin and Markers of Oxidative Stress and Inflammation in a Healthy Population and in Patients with Various Forms of Atherosclerosis. Antioxidants 2022, 11, 2118. [Google Scholar] [CrossRef]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin as a metabolic hormone: The physiological relevance of low levels. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E191–E207. [Google Scholar] [CrossRef]
- Wang, Y.; Chun, O.K.; Song, W.O. Plasma and dietary antioxidant status as cardiovascular disease risk factors: A review of human studies. Nutrients 2013, 5, 2969–3004. [Google Scholar] [CrossRef] [Green Version]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.B.; Crişan, T.O.; Bjornstad, P.; Johnson, R.J. Asymptomatic hyperuricaemia: A silent activator of the innate immune system. Nat. Rev. Rheumatol. 2020, 16, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Bjornstad, P.; Hisatome, I.; Niwa, K.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Jensen, T.; Milagres, T.; Sato, Y.; Garcia, G.; et al. Elevated Serum Uric Acid Level Predicts Rapid Decline in Kidney Function. Am. J. Nephrol. 2017, 45, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwabara, M.; Borghi, C.; Cicero, A.F.G.; Hisatome, I.; Niwa, K.; Ohno, M.; Johnson, R.J.; Lanaspa, M.A. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: A five-year cohort study in Japan. Int. J. Cardiol. 2018, 261, 183–188. [Google Scholar] [CrossRef]
- Kuwabara, M.; Hisatome, I.; Niwa, K.; Hara, S.; Roncal-Jimenez, C.A.; Bjornstad, P.; Nakagawa, T.; Andres-Hernando, A.; Sato, Y.; Jensen, T.; et al. Uric Acid Is a Strong Risk Marker for Developing Hypertension From Prehypertension: A 5-Year Japanese Cohort Study. Hypertension 2018, 71, 78–86. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the Uric Acid Thresholds Predicting an Increased Total and Cardiovascular Mortality Over 20 Years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef]
- Sebekova, K.; Gurecka, R.; Podracka, L. Asymptomatic Hyperuricemia Associates with Cardiometabolic Risk Indicators in Overweight/Obese But Not in Lean Adolescents. Diabetes Metab. Syndr. Obes. 2020, 13, 3977–3992. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Vitamin C: A Review on its Role in the Management of Metabolic Syndrome. Int. J. Med. Sci. 2020, 17, 1625–1638. [Google Scholar] [CrossRef]
- Guo, H.; Ding, J.; Liu, Q.; Li, Y.; Liang, J.; Zhang, Y. Vitamin C and Metabolic Syndrome: A Meta-Analysis of Observational Studies. Front. Nutr. 2021, 8, 728880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, J.; Guo, H.; Liu, Z.; Liu, Q.; Li, Y.; Zhang, D.; Liang, J. Associations of Dietary and Circulating Vitamin E Level with Metabolic Syndrome. A Meta-Analysis of Observational Studies. Front. Nutr. 2021, 8, 783990. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castell, M.; Le Coroller, G.; Landrier, J.F.; Kerkour, D.; Weber, B.; Fagherazzi, G.; Appenzeller, B.M.R.; Vaillant, M.; Bohn, T. Micronutrients and Markers of Oxidative Stress and Inflammation Related to Cardiometabolic Health: Results from the EHES-LUX Study. Nutrients 2020, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, W.Q.; Cao, Y.; He, L.P.; Guan, K.; Ling, W.H.; Chen, Y.M. Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br. J. Nutr. 2014, 112, 2041–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The metabolic syndrome and antioxidant concentrations: Findings from the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senkus, K.E.; Tan, L.; Crowe-White, K.M. Lycopene and Metabolic Syndrome: A Systematic Review of the Literature. Adv. Nutr. 2019, 10, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell. Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef]
- Henning, C.; Glomb, M.A. Pathways of the Maillard reaction under physiological conditions. Glycoconj. J. 2016, 33, 499–512. [Google Scholar] [CrossRef]
- Miyata, T.; van Ypersele de Strihou, C.; Kurokawa, K.; Baynes, J.W. Alterations in non-enzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999, 55, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Monnier, V.M.; Sell, D.R.; Nagaraj, R.H.; Miyata, S.; Grandhee, S.; Odetti, P.; Ibrahim, S.A. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes 1992, 41 (Suppl. 2), 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.H.; Ramasamy, R.; Schmidt, A.M. Advanced Glycation End Products: Building on the Concept of the “Common Soil” in Metabolic Disease. Endocrinology 2020, 161, bqz006. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Shoji, T.; Yokoyama, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Emoto, M.; Shoji, T.; Tamei, H.; Matsuki, H.; et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2587–2593. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Woodward, M.; Tripp, E.; Goldberg, L.; Pyzik, R.; Yee, K.; Tansman, L.; Chen, X.; Mani, V.; et al. Elevated serum advanced glycation end-products in obese indicate risk for the metabolic syndrome: A link between healthy and unhealthy obesity? J. Clin. Endocrinol. Metab. 2015, 100, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Bendayan, M. Renal fate of circulating advanced glycated end products (AGE): Evidence for reabsorption and catabolism of AGE-peptides by renal proximal tubular cells. Diabetologia 1996, 39, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Chen, X.; Li, L.; Li, B.; Yang, Z. The fate of dietary advanced glycation end products in the body: From oral intake to excretion. Crit. Rev. Food Sci. Nutr. 2020, 60, 3475–3491. [Google Scholar] [CrossRef]
- Sebekova, K.; Somoza, V.; Jarcuskova, M.; Heidland, A.; Podracka, L. Plasma advanced glycation end products are decreased in obese children compared with lean controls. Int. J. Pediatr. Obes. 2009, 4, 112–118. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, H.; Mu, D.; Fan, J.; Song, J.; Zhong, Y.; Li, D.; Xia, M. Circulating Retinoic Acid Levels and the Development of Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 1686–1692. [Google Scholar] [CrossRef]
- Choi, K.M.; Ryu, O.H.; Lee, K.W.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, D.S.; Baik, S.H. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res. Clin. Pract. 2007, 75, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid. Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowska, A.M.; Borawska, M.H. Soluble intercellular adhesion molecule-1 (sICAM-1): An overview. Eur. Cytokine Netw. 2004, 15, 91–98. [Google Scholar] [PubMed]
- Timmerman, I.; Daniel, A.E.; Kroon, J.; van Buul, J.D. Chapter Five—Leukocytes Crossing the Endothelium: A Matter of Communication. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 322, pp. 281–329. [Google Scholar]

| CTRL (n = 43) | Pre-MetS (n = 70) | MetS (n = 57) | p | |
|---|---|---|---|---|
| Age, years | 41.7 ± 1.4 | 42.2 ± 1.5 | 42.6 ± 1.4 * | 0.053 |
| Waist circumference, cm | 73.7 ± 5.9 | 83.8 ± 10.8 *** | 89.1 ± 8.9 ***,+++ | <0.001 |
| Body mass index, kg/m2 | 22.1 ± 2.2 | 26.6 ± 4.6 *** | 30.8 ± 4.7 ***,+++ | <0.001 |
| Waist/height | 0.44 ± 0.03 | 0.51 ± 0.06 *** | 0.57 ± 0.06 ***,+++ | <0.001 |
| Systolic BP, mm Hg | 116 ± 7 | 132 ± 14 *** | 136 ± 14 *** | <0.001 |
| Diastolic BP, mm Hg | 74 ± 6 | 83 ± 9 *** | 89 ± 9 ***,++ | <0.001 |
| FPG, mmol/L | 5.0 ± 0.3 | 5.2 ± 0.4 * | 5.6 ± 0.5 ***,+++ | <0.001 |
| FPI, µIU/mL | 7.9 ± 3.5 | 9.5 ± 7.2 | 13.9 ± 8.6 ***,++ | <0.001 |
| QUICKI | 0.356 ± 0.023 | 0.349 ± 0.028 | 0.324 ± 0.021 ***,+++ | <0.001 |
| Total cholesterol, mmol/L | 4.98 ± 0.85 | 5.57 ± 0.88 | 5.35 ± 0.89 | 0.181 |
| HDL-C, mmol/L | 1.76 ± 0.39 | 1.52 ± 0.32 *** | 1.22 ± 0.26 ***,++ | <0.001 |
| Triacylglycerols, mmol/L | 0.85 ± 0.29 | 1.14 ± 0.46 * | 1.84 ± 0.69 ***,+++ | <0.001 |
| AIP | −0.33 ± 0.17 | −0.14 ± 0.20 *** | 0.16 ± 0.20 ***,+++ | <0.001 |
| eGFR, mL/min/1.73 m2 | 1.5 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.3 | 0.363 |
| CTRL (n = 43) | Pre-MetS (n = 70) | MetS (n = 57) | p | |
|---|---|---|---|---|
| Waist/height ≥ 0.5 | NA | 32 (45.7) | 53 (93.0) | <0.001 |
| Systolic BP ≥ 130 mm Hg | NA | 40 (57.1) | 46 (80.7) | 0.007 |
| FPG ≥ 5.6 mmol/L | NA | 13 (18.6) | 34 (59.6) | <0.001 |
| HDL-C < 1.3 mmol/L | NA | 14 (20.0) | 36 (63.2) | <0.001 |
| Triacylglycerols ≥ 1.7 mmol/L | NA | 5 (7.1) | 30 (52.6) | <0.001 |
| BMI > 29.9. kg/m2 | 0 | 14 (20.0) | 33 (57.9) | <0.001 |
| Diastolic BP ≥ 85 mm Hg | 3 (7.0) | 29 (41.4) | 40 (70.2) | <0.001 |
| FPI ≥ 20 µIU/mL | 0 | 2 (2.9) | 8 (14.0) | 0.005 |
| AIP ≥ 0.11 | 0 | 8 (11.4) | 35 (61.4) | <0.001 |
| Uric acid > 340 µmol/L | 0 | 1 (1.4) | 5 (8.8) | 0.029 |
| C-reactive protein > 3 mg/L | 21 (48.8) | 47 (67.1) | 51 (89.5) | <0.001 |
| CTRL (n = 43) | Pre-MetS (n = 70) | MetS (n = 57) | p | |
|---|---|---|---|---|
| Superoxide dismutase, U/g Hb | 1574 ± 452 | 1632 ± 386 | 1652 ± 391 | 0.628 |
| Catalase, kU/g Hb | 11.8 ± 4.7 | 11.5 ± 4.4 | 11.4 ± 5.4 | 0.929 |
| Glutathione peroxidase, U/g Hb | 10.0 ± 3.8 | 9.4 ± 4.3 | 11.0 ± 3.6 | 0.082 |
| Glutathione-S-transferase, U/g Hb | 44.2 ± 8.7 | 44.1 ± 9.7 | 44.2 ± 9.4 | 0.998 |
| Ceruloplasmin oxidase, U/L | 89 ± 44 | 103 ± 35 | 97 ± 30 | 0.145 |
| Albumin, g/L | 44.9 ± 3.3 | 45.1 ± 4.2 | 44.3 ± 3.5 | 0.529 |
| Total bilirubin, µmol/L | 10.0 ± 5.9 | 9.4 ± 4.7 | 7.7 ± 3.8 * | 0.033 |
| Uric acid, µmol/L | 214 ± 54 | 239 ± 49 | 262 ± 64 *** | <0.001 |
| CTRL (n = 43) | Pre-MetS (n = 70) | MetS (n = 57) | p | |
|---|---|---|---|---|
| Vitamin C, µmol/L | 49.3 ± 19.4 | 44.8 ± 21.0 | 46.8 ± 20.3 | 0.528 |
| Retinol, µmol/L | 2.32 ± 0.67 | 2.52 ± 0.81 | 2.55 ± 0.81 | 0.286 |
| ß-carotene, µmol/L | 1.20 ± 0.73 | 1.01 ± 0.60 | 0.86 ± 0.59 * | 0.032 |
| Lycopene, µmol/L | 0.50 ± 0.28 | 0.43 ± 0.20 | 0.47 ± 0.24 | 0.353 |
| Xanthophyll, µmol/L | 0.30 ± 0.15 | 0.25 ± 0.16 | 0.22 ± 0.13 * | 0.022 |
| Carotenoids, µmol/L | 4.32 ± 1.21 | 4.22 ± 1.06 | 4.10 ± 1.22 | 0.630 |
| Carotenoids/lipids, µmol/mmol | 0.75 ± 0.21 | 0.68 ± 0.23 | 0.59 ± 0.19 ***,+ | <0.001 |
| α-tocopherol, µmol/L | 26.6 ± 7.2 | 27.4 ± 6.3 | 31.3 ± 7.8 **,++ | 0.001 |
| γ-tocopherol, µmol/L | 1.09 ± 0.59 | 1.14 ± 0.55 | 1.33 ± 0.65 | 0.088 |
| Tocopherols, µmol/L | 27.7 ± 7.5 | 28.6 ± 6.4 | 32.6 ± 8.0 **,++ | 0.001 |
| Tocopherols/lipids, µmol/mmol | 4.8 ± 1.2 | 4.6 ± 1.0 | 4.6 ± 1.0 | 0.612 |
| Cysteine, µmol/L | 225 ± 40 | 220 ± 40 | 207 ± 42 | 0.081 |
| GSH, µmol/L | 9.1 ± 2.2 | 8.3 ± 2.6 | 7.9 ± 2.4 * | 0.041 |
| CTRL (n = 43) | Pre-MetS (n = 70) | MetS (n = 57) | p | |
|---|---|---|---|---|
| Leukocytes, 109/L | 6.0 ± 1.4 | 6.9 ± 1.6 * | 7.1 ± 1.7 ** | 0.002 |
| C-reactive protein, mg/L | 3.2 ± 1.5 | 4.7 ± 2.8 ** | 5.4 ± 2.1 *** | <0.001 |
| Interleukine-6, pg/mL | 0.2 (0.1; 0.6) | 0.4 (0.1; 1.6) * | 0.4 (0.1; 1.7) * | 0.014 |
| TNF-α, pg/mL | 5.2 ± 2.2 | 5.0 ± 2.6 | 5.2 ± 2.7 | 0.927 |
| Interleukine-10, pg/mL | 2.0 (0.8; 4.9) | 3.3 (1.5; 7.5) ** | 2.6 (1.2; 5.9) | 0.006 |
| sE-selectin, ng/mL | 47.2 ± 22.8 | 57.2 ± 30.2 | 66.3 ± 32.1 ** | 0.006 |
| sICAM-1, ng/mL | 152 ± 26 | 152 ± 27 | 150 ± 24 | 0.903 |
| sVAP-1, ng/mL | 262 ± 77 | 260 ± 80 | 257 ± 75 | 0.953 |
| sRAGE, pg/mL | 1683 ± 573 | 1412 ± 402 * | 1373 ± 428 * | 0.010 |
| WHtR | FPG | TAG | HDL-C | SBP | |
|---|---|---|---|---|---|
| C-reactive protein | 1.54 | 1.20 | 1.38 | 1.23 | 1.26 |
| Uric acid | 1.35 | 1.46 | 1.26 | 1.34 | 1.40 |
| sRAGE | 1.28 | 1.46 | 1.09 | 0.92 | 0.59 |
| Carotenoids/lipids | 1.28 | 1.83 | 1.66 | 0.87 | 0.88 |
| Interleukine-6 | 1.13 | 1.17 | 1.13 | 1.28 | 1.09 |
| Leukocytes | 1.10 | 0.95 | 1.26 | 1.06 | 1.01 |
| E-selectin | 0.86 | 0.85 | 1.07 | 1.73 | 1.34 |
| Interleukine-10 | 0.80 | 0.78 | 0.59 | 0.46 | 0.70 |
| Glutathione peroxidase | 0.64 | 0.33 | 0.42 | 0.75 | 0.96 |
| GSH | 0.63 | 0.93 | 0.67 | 0.64 | 1.09 |
| Cysteine | 0.62 | 0.64 | 0.44 | 0.43 | 1.20 |
| SSAO | 0.55 | 0.65 | 0.49 | 0.72 | 0.28 |
| Total bilirubin | 0.45 | 0.75 | 0.35 | 0.66 | 0.47 |
| R2 | 0.50 | 0.17 | 0.27 | 0.16 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šebeková, K.; Staruchová, M.; Mišľanová, C.; Líšková, A.; Horváthová, M.; Tulinská, J.; Lehotská Mikušová, M.; Szabová, M.; Gurecká, R.; Koborová, I.; et al. Association of Inflammatory and Oxidative Status Markers with Metabolic Syndrome and Its Components in 40-to-45-Year-Old Females: A Cross-Sectional Study. Antioxidants 2023, 12, 1221. https://doi.org/10.3390/antiox12061221
Šebeková K, Staruchová M, Mišľanová C, Líšková A, Horváthová M, Tulinská J, Lehotská Mikušová M, Szabová M, Gurecká R, Koborová I, et al. Association of Inflammatory and Oxidative Status Markers with Metabolic Syndrome and Its Components in 40-to-45-Year-Old Females: A Cross-Sectional Study. Antioxidants. 2023; 12(6):1221. https://doi.org/10.3390/antiox12061221
Chicago/Turabian StyleŠebeková, Katarína, Marta Staruchová, Csilla Mišľanová, Aurélia Líšková, Mira Horváthová, Jana Tulinská, Miroslava Lehotská Mikušová, Michaela Szabová, Radana Gurecká, Ivana Koborová, and et al. 2023. "Association of Inflammatory and Oxidative Status Markers with Metabolic Syndrome and Its Components in 40-to-45-Year-Old Females: A Cross-Sectional Study" Antioxidants 12, no. 6: 1221. https://doi.org/10.3390/antiox12061221
APA StyleŠebeková, K., Staruchová, M., Mišľanová, C., Líšková, A., Horváthová, M., Tulinská, J., Lehotská Mikušová, M., Szabová, M., Gurecká, R., Koborová, I., Csongová, M., Tábi, T., Szökö, É., & Volkovová, K. (2023). Association of Inflammatory and Oxidative Status Markers with Metabolic Syndrome and Its Components in 40-to-45-Year-Old Females: A Cross-Sectional Study. Antioxidants, 12(6), 1221. https://doi.org/10.3390/antiox12061221