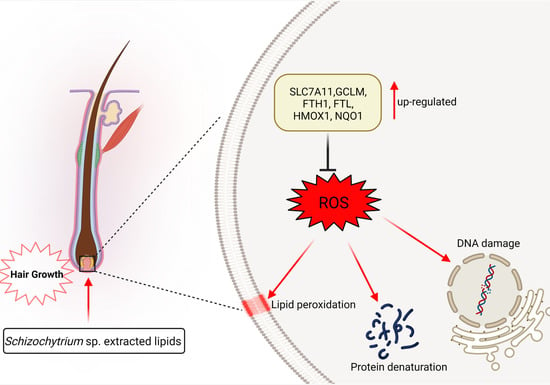
Schizochytrium sp. Extracted Lipids Prevent Alopecia by Enhancing Antioxidation and Inhibiting Ferroptosis of Dermal Papilla Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Composition Analysis of SEL
2.3. Cell Viability Assay
2.4. Antioxidant Capacity Assay
2.5. 2′,7′-Dichlorodihydrofluorescein Diacetate Assay for Detecting the ROS Level
2.6. RNA-Sequencing Analysis
2.7. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.8. Western Blot Analysis
2.9. Hair Growth Activity In Vivo
2.10. Morphological Analysis of Hair Follicles by HE Stains
2.11. Statistical Analysis
3. Results
3.1. Composition Analysis of SEL
3.2. SEL Promoted the Proliferation of DPC
3.3. SEL Enhanced the Antioxidant Capacity of DPC
3.4. Comparative Transcriptome Analysis for Various Treatment
3.4.1. Quality Analysis of Transcriptome Data and Statistics of DEGs
3.4.2. Functional Analysis by GO and KEGG Enrichment
3.5. Effects of SEL on the Proliferation and Antioxidation of DPC
3.5.1. Changes in the Ferroptosis Signaling Pathway in DPC after SEL Treatment
3.5.2. Effect of SEL on the Antioxidant Capacity of DPC
3.6. SEL Promoted Hair Growth in C57BL/6 Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamerson, T.A.; Aguh, C. An Approach to Patients with Alopecia. Med. Clin. N. Am. 2021, 105, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Mirmirani, P. Clinical approach to the patient with alopecia. Semin. Cutan. Med. Surg. 2006, 25, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell. Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Akar, A.; Arca, E.; Erbil, H.; Akay, C.; Sayal, A.; Gür, A.R. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J. Dermatol. Sci. 2002, 29, 85–90. [Google Scholar] [CrossRef]
- Bakry, O.A.; Elshazly, R.M.; Shoeib, M.A.; Gooda, A. Oxidative stress in alopecia areata: A case-control study. Am. J. Clin. Dermatol. 2014, 15, 57–64. [Google Scholar] [CrossRef]
- Yenin, J.Z.; Serarslan, G.; Yönden, Z.; Ulutaş, K.T. Investigation of oxidative stress in patients with alopecia areata and its relationship with disease severity, duration, recurrence and pattern. Clin. Exp. Dermatol. 2015, 40, 617–621. [Google Scholar] [CrossRef]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A. 5% Minoxidil: Treatment for female pattern hair loss. Skin. Ther. Lett. 2014, 19, 5–7. [Google Scholar]
- Motofei, I.G.; Rowland, D.L.; Tampa, M.; Sarbu, M.I.; Mitran, M.I.; Mitran, C.I.; Stoian, A.P.; Diaconu, C.C.; Paunica, S.; Georgescu, S.R. Finasteride and androgenic alopecia; from therapeutic options to medical implications. J. Dermatolog Treat. 2020, 31, 415–421. [Google Scholar] [CrossRef]
- Goren, A.; Naccarato, T. Minoxidil in the treatment of androgenetic alopecia. Dermatol. Ther. 2018, 31, e12686. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Said, M.A.; Mehta, A. The Impact of 5α-Reductase Inhibitor Use for Male Pattern Hair Loss on Men’s Health. Curr. Urol. Rep. 2018, 19, 65. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia 2016, 114, 18–25. [Google Scholar] [CrossRef]
- Junlatat, J.; Sripanidkulchai, B. Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytother. Res. 2014, 28, 1030–1036. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, J.M.; Park, S.Y.; Yang, J.E.; Kim, J.H.; Yi, T.H. Hair growth activity of Crataegus pinnatifida on C57BL/6 mouse model. Phytother. Res. 2013, 27, 1352–1357. [Google Scholar] [CrossRef]
- Boisvert, W.A.; Yu, M.; Choi, Y.; Jeong, G.H.; Zhang, Y.L.; Cho, S.; Choi, C.; Lee, S.; Lee, B.H. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complement. Altern. Med. 2017, 17, 109. [Google Scholar] [CrossRef]
- Sun, Y.N.; Cui, L.; Li, W.; Yan, X.T.; Yang, S.Y.; Kang, J.I.; Kang, H.K.; Kim, Y.H. Promotion effect of constituents from the root of Polygonum multiflorum on hair growth. Bioorg. Med. Chem. Lett. 2013, 23, 4801–4805. [Google Scholar] [CrossRef]
- Kwon, T.R.; Oh, C.T.; Choi, E.J.; Park, H.M.; Han, H.J.; Ji, H.J.; Kim, B.J. Human placental extract exerts hair growth-promoting effects through the GSK-3β signaling pathway in human dermal papilla cells. Int. J. Mol. Med. 2015, 36, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Moon, J.S.; Jeon, B.S.; Jeon, Y.J.; Yoon, B.I.; Lim, C.J. Hair growth promoting potential of phospholipids purified from porcine lung tissues. Biomol. Ther. 2015, 23, 174–179. [Google Scholar] [CrossRef]
- Miyata, M.; Iwata, S.; Mifude, C.K.; Tajima, M.; Kameyama, M.; Ihara, M.; Matsui, T.; Yamagishi, S.I.; Ishitobi, H.; Miyaki, S.; et al. A Novel Mucidosphaerium sp. Downregulates Inflammatory Gene Expression in Skin and Articular Cells. Altern. Ther. Health Med. 2021, 27, 28–34. [Google Scholar] [PubMed]
- Bak, S.S.; Ahn, B.N.; Kim, J.A.; Shin, S.H.; Kim, J.C.; Kim, M.K.; Sung, Y.K.; Kim, S.K. Ecklonia cava promotes hair growth. Clin. Exp. Dermatol. 2013, 38, 904–910. [Google Scholar] [CrossRef]
- Bak, S.S.; Sung, Y.K.; Kim, S.K. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn-Schmiedebergs Arch. Pharmacol. 2014, 387, 789–793. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Sasaki, S.; Hozumi, Y.; Kondo, S. Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Exp. Dermatol. 2005, 14, 323–328. [Google Scholar] [CrossRef]
- Khidhir, K.G.; Woodward, D.F.; Farjo, N.P.; Farjo, B.K.; Tang, E.S.; Wang, J.W.; Picksley, S.M.; Randall, V.A. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013, 27, 557–567. [Google Scholar] [CrossRef]
- Kang, J.I.; Yoon, H.S.; Kim, S.M.; Park, J.E.; Hyun, Y.J.; Ko, A.; Ahn, Y.S.; Koh, Y.S.; Hyun, J.W.; Yoo, E.S.; et al. Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways. Int. J. Mol. Sci. 2018, 19, 2770. [Google Scholar] [CrossRef]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The discovery and early structural studies of arachidonic acid. J. Lipid Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef]
- Munkhbayar, S.; Jang, S.; Cho, A.R.; Choi, S.J.; Shin, C.Y.; Eun, H.C.; Kim, K.H.; Kwon, O. Role of Arachidonic Acid in Promoting Hair Growth. Ann. Dermatol. 2016, 28, 55–64. [Google Scholar] [CrossRef]
- Ryu, H.S.; Jeong, J.; Lee, C.M.; Lee, K.S.; Lee, J.N.; Park, S.M.; Lee, Y.M. Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed. Molecules 2021, 26, 2117. [Google Scholar] [CrossRef]
- Bao, Z.; Zhu, Y.; Feng, Y.; Zhang, K.; Zhang, M.; Wang, Z.; Yu, L. Enhancement of lipid accumulation and docosahexaenoic acid synthesis in Schizochytrium sp. H016 by exogenous supplementation of sesamol. Bioresour. Technol. 2022, 345, 126527. [Google Scholar] [CrossRef]
- Liang, L.; Zheng, X.; Fan, W.; Chen, D.; Huang, Z.; Peng, J.; Zhu, J.; Tang, W.; Chen, Y.; Xue, T. Genome and Transcriptome Analyses Provide Insight Into the Omega-3 Long-Chain Polyunsaturated Fatty Acids Biosynthesis of Schizochytrium limacinum SR21. Front. Microbiol. 2020, 11, 687. [Google Scholar] [CrossRef]
- Liang, S.T.; Yang, X.W.; Zhu, X.Y.; Ibrar, M.; Liu, L.X.; Li, S.T.; Li, X.; Tian, T.; Li, S.F. Metabolic Engineering to Improve Docosahexaenoic Acid Production in Marine Protist Aurantiochytrium sp. by Disrupting 2,4-Dienoyl-CoA Reductase. Front. Mar. Sci. 2022, 9, 939716. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, Y.H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 144. [Google Scholar] [CrossRef]
- Bejaoui, M.; Taarji, N.; Saito, M.; Nakajima, M.; Isoda, H. Argan (Argania Spinosa) press cake extract enhances cell proliferation and prevents oxidative stress and inflammation of human dermal papilla cells. J. Dermatol. Sci. 2021, 103, 33–40. [Google Scholar] [CrossRef]
- Ni, S.; Qian, Z.; Yuan, Y.; Li, D.; Zhong, Z.; Ghorbani, F.; Zhang, X.; Zhang, F.; Zhang, Z.; Liu, Z.; et al. Schisandrin A restrains osteoclastogenesis by inhibiting reactive oxygen species and activating Nrf2 signalling. Cell. Prolif. 2020, 53, e12882. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sun, K.; Yu, S.; Luo, J.; Guo, J.; Lin, J.; Wang, G.; Guo, Z.; Ye, Y.; Guo, F. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Transl. 2021, 27, 33–43. [Google Scholar] [CrossRef]
- Mustafa, A.I.; Khashaba, R.A.; Fawzy, E.; Baghdady, S.M.A.; Rezk, S.M. Cross talk between oxidative stress and inflammation in alopecia areata. J. Cosmet. Dermatol. 2021, 20, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. The impact of oxidative stress on hair. Int. J. Cosmet. Sci. 2015, 37 (Suppl. 2), 25–30. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichol. 2009, 1, 6–14. [Google Scholar] [CrossRef]
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef]
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The Application of Ferroptosis in Diseases. Pharmacol. Res. 2020, 159, 104919. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc⁻ cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, J.; Hao, X.; Li, H.; Zhang, G.; Liu, X.; Li, X.; Zhao, C.; Kuang, W.; Chen, D.; et al. FTH1 Inhibits Ferroptosis Through Ferritinophagy in the 6-OHDA Model of Parkinson’s Disease. Neurotherapeutics 2020, 17, 1796–1812. [Google Scholar] [CrossRef]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress and its impact on skin, scalp and hair. Int. J. Cosmet. Sci. 2021, 43 (Suppl. 1), S9–S13. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Meister, A. Selective modification of glutathione metabolism. Science 1983, 220, 472–477. [Google Scholar] [CrossRef]
- Homma, T.; Fujii, J. Application of Glutathione as Anti-Oxidative and Anti-Aging Drugs. Curr. Drug. Metab. 2015, 16, 560–571. [Google Scholar] [CrossRef]
- Oh, E.T.; Park, H.J. Implications of NQO1 in cancer therapy. BMB Rep. 2015, 48, 609–617. [Google Scholar] [CrossRef]
- Singh, S.; Patil, A.; Kianfar, N.; Waśkiel-Burnat, A.; Rudnicka, L.; Sinclair, R.; Goldust, M. Does topical minoxidil at concentrations higher than 5% provide additional clinical benefit? Clin. Exp. Dermatol. 2022, 47, 1951–1955. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R. Melanogenesis is coupled to murine anagen: Toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J. Investig. Dermatol. 1993, 101, 90s–97s. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Mathur, M.C. Oxidative stress in alopecia areata: A systematic review and meta-analysis. Int. J. Dermatol. 2020, 59, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kaya Erdogan, H.; Bulur, I.; Kocaturk, E.; Yildiz, B.; Saracoglu, Z.N.; Alatas, O. The role of oxidative stress in early-onset androgenetic alopecia. J. Cosmet. Dermatol. 2017, 16, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Tonegawa, S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef]
- Hunt, R.C.; Handy, I.; Smith, A. Heme-mediated reactive oxygen species toxicity to retinal pigment epithelial cells is reduced by hemopexin. J. Cell. Physiol. 1996, 168, 81–86. [Google Scholar]
- Vile, G.F.; Basu-Modak, S.; Waltner, C.; Tyrrell, R.M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc. Natl. Acad. Sci. USA 1994, 91, 2607–2610. [Google Scholar] [CrossRef]
- Zakhary, R.; Gaine, S.P.; Dinerman, J.L.; Ruat, M.; Flavahan, N.A.; Snyder, S.H. Heme oxygenase 2: Endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl. Acad. Sci. USA 1996, 93, 795–798. [Google Scholar] [CrossRef]
- Yun, S.J.; Kim, H.S.; Choi, J.Y.; Lee, J.B.; Kim, S.J.; Won, Y.H.; Lee, S.C. Decreased heme oxygenase-1 expression in the scalp of patients with alopecia areata: The pathogenic role of heme oxygenase-1. J. Dermatol. Sci. 2009, 54, 43–45. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef]
- Jo, H.S.; Kim, D.S.; Ahn, E.H.; Kim, D.W.; Shin, M.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; Yeo, E.J.; Choi, Y.J.; et al. Protective effects of Tat-NQO1 against oxidative stress-induced HT-22 cell damage, and ischemic injury in animals. BMB Rep. 2016, 49, 617–622. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Hong, J.; Tang, A.; Liu, Y.; Xie, N.; Nie, G.; Yan, X.; Liang, M. Biochemistry of mammalian ferritins in the regulation of cellular iron homeostasis and oxidative responses. Sci. China Life Sci. 2021, 64, 352–362. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Ren, J.X.; Li, C.; Yan, X.L.; Qu, Y.; Yang, Y.; Guo, Z.N. Crosstalk between Oxidative Stress and Ferroptosis/Oxytosis in Ischemic Stroke: Possible Targets and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6643382. [Google Scholar] [CrossRef]
- Guo, J.; Xu, B.; Han, Q.; Zhou, H.; Xia, Y.; Gong, C.; Dai, X.; Li, Z.; Wu, G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 2018, 50, 445–460. [Google Scholar] [CrossRef]
- Niki, E. Lipid oxidation in the skin. Free. Radic. Res. 2015, 49, 827–834. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Adebayo, A.A.; Onaolapo, O.J. Oral phenytoin protects against experimental cyclophosphamide-chemotherapy induced hair loss. Pathophysiology 2018, 25, 31–39. [Google Scholar] [CrossRef]
- Naito, A.; Midorikawa, T.; Yoshino, T.; Ohdera, M. Lipid peroxides induce early onset of catagen phase in murine hair cycles. Int. J. Mol. Med. 2008, 22, 725–729. [Google Scholar]
- Dierge, E.; Debock, E.; Guilbaud, C.; Corbet, C.; Mignolet, E.; Mignard, L.; Bastien, E.; Dessy, C.; Larondelle, Y.; Feron, O. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell. Metab. 2021, 33, 1701–1715.e1705. [Google Scholar] [CrossRef]
- Nassar, Z.D.; Mah, C.Y.; Dehairs, J.; Burvenich, I.J.; Irani, S.; Centenera, M.M.; Helm, M.; Shrestha, R.K.; Moldovan, M.; Don, A.S.; et al. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. Elife 2020, 9, e54166. [Google Scholar] [CrossRef]
- Lee, J.Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.J.; et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Muhoberac, B.B.; Vidal, R. Iron, Ferritin, Hereditary Ferritinopathy, and Neurodegeneration. Front. Neurosci. 2019, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wu, Q.; Guo, X.; Zhang, Z.; Shen, Y.; Wang, F. MBD5 regulates iron metabolism via methylation-independent genomic targeting of Fth1 through KAT2A in mice. Br. J. Haematol. 2014, 166, 279–291. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, S.H.; Park, H.R.; Lee, Y.; Kang, H.; Kim, J.E. Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles. Int. J. Mol. Sci. 2021, 22, 4581. [Google Scholar] [CrossRef]
- Lee, S.A.; Li, K.N.; Tumbar, T. Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Exp. Dermatol. 2021, 30, 430–447. [Google Scholar] [CrossRef]
- Ceruti, J.M.; Leirós, G.J.; Balañá, M.E. Androgens and androgen receptor action in skin and hair follicles. Mol. Cell. Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Wang, B.; Ibrar, M.; Ying, M.; Li, S.; Yang, X. Schizochytrium sp. Extracted Lipids Prevent Alopecia by Enhancing Antioxidation and Inhibiting Ferroptosis of Dermal Papilla Cells. Antioxidants 2023, 12, 1332. https://doi.org/10.3390/antiox12071332
Zeng Z, Wang B, Ibrar M, Ying M, Li S, Yang X. Schizochytrium sp. Extracted Lipids Prevent Alopecia by Enhancing Antioxidation and Inhibiting Ferroptosis of Dermal Papilla Cells. Antioxidants. 2023; 12(7):1332. https://doi.org/10.3390/antiox12071332
Chicago/Turabian StyleZeng, Zuye, Boyu Wang, Muhammad Ibrar, Ming Ying, Shuangfei Li, and Xuewei Yang. 2023. "Schizochytrium sp. Extracted Lipids Prevent Alopecia by Enhancing Antioxidation and Inhibiting Ferroptosis of Dermal Papilla Cells" Antioxidants 12, no. 7: 1332. https://doi.org/10.3390/antiox12071332
APA StyleZeng, Z., Wang, B., Ibrar, M., Ying, M., Li, S., & Yang, X. (2023). Schizochytrium sp. Extracted Lipids Prevent Alopecia by Enhancing Antioxidation and Inhibiting Ferroptosis of Dermal Papilla Cells. Antioxidants, 12(7), 1332. https://doi.org/10.3390/antiox12071332