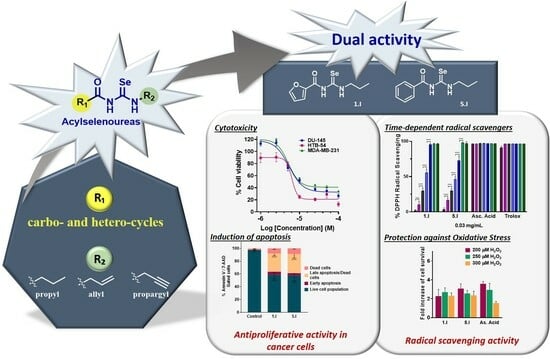
Novel Acylselenourea Derivatives: Dual Molecules with Anticancer and Radical Scavenging Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. General Information
2.1.2. General Procedure for the Preparation of Acylselenourea Derivatives
2.2. Radical Scavenging Activity
DPPH Radical Scavenging Assay
2.3. Biological Evaluation
2.3.1. Cell Culture Conditions
2.3.2. Cell Viability Assay
2.3.3. Protective Effects against H2O2-Induced Oxidative Stress in DU-145 Cells
- Cells without compounds, only treated with H2O2:
- Cells treated with compounds and H2O2:
2.3.4. Apoptosis Assays
2.3.5. ROS Measurement
2.3.6. Statistical Analysis
2.4. X-ray Christallography of 1.II
3. Results
3.1. Design
3.2. Chemistry
3.3. Antioxidant Activity
DPPH Radical Scavenging Assay
3.4. Biological Evaluation
3.4.1. Cytotoxic Activity
- Compounds 5.I and 6.I differ only in the presence of a double bond (6.I) between the benzene ring and the carbonyl group. This structural modification led to a loss of activity against MDA-MB-231, HT-29, and HTB-54 cell lines, whereas it improved the activity against the prostate DU-145 cell line (cell growth at 10 µM of 42.4% and 19.47% for 5.I and 6.I, respectively). For these same derivatives, but of the series II (allyl), the introduction of this double bond was also observed to slightly improve the activity in the MDA-MB-231 cell line (cell growth at 10 µM of 80.4% and 51.8% for 5.II and 6.II, respectively). Compound 6.II showed better activity than 5.II against the DU-145 cell line (cell growth at 10 µM of 57.30% and 97.5%, respectively);
- On the other hand, comparing compounds 5.I, 5.II, and 5.III, which only differ in the aliphatic chain, it was observed that 5.I (propyl) showed a more potent anticancer activity against the panel of the four cell lines, 5.II and 5.III being hardly active;
- Compounds 1.II and 3.II contain a furan ring in their structure and their only difference is the double bond (3.II) between the heterocycle and carbonyl. However, on this occasion, both showed low activity with cell growth % between 54.8–95.0 at 10 µM. Their series III analogues (1.III and 3.III) also showed low-moderate activity for the MDA-MB-231, HT-29, and HTB-54 cell lines. However, the DU-145 cell line proved to be more sensitive to 1.III, showing a cell growth of 42.2 at 10 µM;
- Compound 1.I (propyl) showed more potent anticancer activity than its analogues (1.II and 1.III) against the same four cancer cell lines panel. On the other hand, compounds 3.II and 3.III did not exhibit great anticancer activity;
- For the thiophene derivatives (2.II and 4.II), the presence of this double bond led to a loss of activity in the lung HTB-54 cell line (cell growth at 10 µM of 35.8% and 79.2% for 2.II and 4.II, respectively). For the rest of the cell lines, 2.II and 4.II presented moderate-low activity. The presence of the allyl chain in the thiophene analogues (2.II) led to a loss of activity compared to propyl and propargyl derivatives (2.I and 2.III);
- Compounds 8.II and 9.II are allylic derivatives of benzodioxole that differ in the presence of the double bond between the core and the carbonyl. However, this small structural modification does not seem to affect the anticancer activity against MDA-MB-231, HT-29, and DU-145 cancer cell lines. Molecule 8.II, the derivative presenting the benzodioxole core directly attached to the carbonyl, was shown to be more potent against the HTB-54 cell line (cell growth at 10 µM of 51.8% and 82.8% for 8.II and 9.II, respectively).
3.4.2. Protective Effects against H2O2-Induced Cell Damage in DU-145 Cells
3.4.3. Compounds 1.I and 5.I Induce Apoptosis in DU-145 Cells
3.4.4. Compounds 1.I and 5.I Slightly Induce ROS Production during 24 h of Treatment
3.5. X-ray Crystallography of N-(allylcarbamoselenoyl)furan-2-carboxamide (Compound 1.II)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| DMSO | Dimethylsulfoxide |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| N,N-DMF | N,N-dimethylformamide |
| NMR | Nuclear Magnetic Resonance |
| NP | Natural Products |
| PS | Phosphotidylserine |
| RDA | Recommended Dietary Allowances |
| ROS | Reactive Oxygen Species |
| Se | Selenium |
| SI | Selectivity Index |
| TLC | thin-layer chromatography |
References
- Ferlay, J.E.M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 10 March 2023).
- Bartolini, D.; Sancineto, L.; Fabro de Bem, A.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in Cancer Therapy: An Overview. Adv. Cancer Res. 2017, 136, 259–302. [Google Scholar] [CrossRef]
- Diamond, A.M. Selenoproteins of the Human Prostate: Unusual Properties and Role in Cancer Etiology. Biol. Trace Elem. Res. 2019, 192, 51–59. [Google Scholar] [CrossRef]
- Assi, M. The differential role of reactive oxygen species in early and late stages of cancer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R646–R653. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive Oxygen and Nitrogen Species-Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Short, S.P.; Williams, C.S. Selenoproteins in Tumorigenesis and Cancer Progression. Adv. Cancer Res. 2017, 136, 49–83. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Soltani, A.; Ghahremanloo, A.; Javid, H.; Hashemy, S.I. The thioredoxin system and cancer therapy: A review. Cancer Chemother. Pharm. 2019, 84, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free. Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Garnica, P.; Encío, I.; Plano, D.; Palop, J.A.; Sanmartín, C. Combined Acylselenourea-Diselenide Structures: New Potent and Selective Antitumoral Agents as Autophagy Activators. ACS Med. Chem. Lett. 2018, 9, 306–311. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Ramos-Inza, S.; Aydillo, C.; Talavera, I.; Encío, I.; Plano, D.; Sanmartín, C. Novel N,N’-Disubstituted Acylselenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2020, 9, 55. [Google Scholar] [CrossRef]
- Romero-Hernández, L.L.; Merino-Montiel, P.; Montiel-Smith, S.; Meza-Reyes, S.; Vega-Báez, J.L.; Abasolo, I.; Schwartz, S., Jr.; López, Ó.; Fernández-Bolaños, J.G. Diosgenin-based thio(seleno)ureas and triazolyl glycoconjugates as hybrid drugs. Antioxidant and antiproliferative profile. Eur. J. Med. Chem. 2015, 99, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.A.R.; Siminski, T.; Canto, R.F.S.; Almeida, G.M.; Mota, N.; Ourique, F.; Pedrosa, R.C.; Braga, A.L. Novel pyrimidinic selenourea induces DNA damage, cell cycle arrest, and apoptosis in human breast carcinoma. Eur. J. Med. Chem. 2018, 155, 503–515. [Google Scholar] [CrossRef]
- Svinyarov, I.; Bogdanov, M.G. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. Eur. J. Med. Chem. 2014, 78, 198–206. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Astrain-Redin, N.; Talavera, I.; Moreno, E.; Ramírez, M.J.; Martínez-Sáez, N.; Encío, I.; Sharma, A.K.; Sanmartín, C.; Plano, D. Seleno-Analogs of Scaffolds Resembling Natural Products a Novel Warhead toward Dual Compounds. Antioxidants 2023, 12, 139. [Google Scholar] [CrossRef]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine’s Tool Box. Molecules 2015, 20, 16852–16891. [Google Scholar] [CrossRef]
- Pearce, S. The Importance of Heterocyclic Compounds in Anti-Cancer Drug Design. Drug Discovery World, 2017. [Google Scholar]
- Astrain-Redin, N.; Sanmartin, C.; Sharma, A.K.; Plano, D. From Natural Sources to Synthetic Derivatives: The Allyl Motif as a Powerful Tool for Fragment-Based Design in Cancer Treatment. J. Med. Chem. 2023, 66, 3703–3731. [Google Scholar] [CrossRef] [PubMed]
- Hitora, Y.; Takada, K.; Okada, S.; Ise, Y.; Matsunaga, S. (-)-Duryne and its homologues, cytotoxic acetylenes from a marine Sponge Petrosia sp. J. Nat. Prod. 2011, 74, 1262–1267. [Google Scholar] [CrossRef]
- Simmons, T.L.; Engene, N.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, L.; Gerwick, W.H. Viridamides A and B, lipodepsipeptides with antiprotozoal activity from the marine cyanobacterium Oscillatoria nigro-viridis. J. Nat. Prod. 2008, 71, 1544–1550. [Google Scholar] [CrossRef]
- Hua, G.; Cordes, D.B.; Du, J.; Slawin, A.M.Z.; Woollins, J.D. Diverse Derivatives of Selenoureas: A Synthetic and Single Crystal Structural Study. Molecules 2018, 23, 2143. [Google Scholar] [CrossRef]
- Angeli, A.; Carta, F.; Bartolucci, G.; Supuran, C.T. Synthesis of novel acyl selenoureido benzensulfonamides as carbonic anhydrase I, II, VII and IX inhibitors. Bioorg. Med. Chem. 2017, 25, 3567–3573. [Google Scholar] [CrossRef]
- Angeli, A.; Peat, T.S.; Bartolucci, G.; Nocentini, A.; Supuran, C.T.; Carta, F. Intramolecular oxidative deselenization of acylselenoureas: A facile synthesis of benzoxazole amides and carbonic anhydrase inhibitors. Org. Biomol. Chem. 2016, 14, 11353–11356. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharm. 2003, 57, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds--from toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Estrada, M.; Herrera-Arozamena, C.; Pérez, C.; Viña, D.; Romero, A.; Morales-García, J.A.; Pérez-Castillo, A.; Rodríguez-Franco, M.I. New cinnamic-N-benzylpiperidine and cinnamic-N,N-dibenzyl(N-methyl)amine hybrids as Alzheimer-directed multitarget drugs with antioxidant, cholinergic, neuroprotective and neurogenic properties. Eur. J. Med. Chem. 2016, 121, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Cetintas, V.B.; Kucukaslan, A.S.; Kosova, B.; Tetik, A.; Selvi, N.; Cok, G.; Gunduz, C.; Eroglu, Z. Cisplatin resistance induced by decreased apoptotic activity in non-small-cell lung cancer cell lines. Cell Biol. Int. 2012, 36, 261–265. [Google Scholar] [CrossRef]
- Davou, G.I.; Chuwang, N.J.; Essien, U.C.; Choji, T.P.P.; Echeonwu, B.C.; Lugos, M.D. Cytotoxicity analysis of etoposide and cisplatin on cell lines from human lung cancer and normal human lung. Int. Res. J. Med. Med. Sci. 2019, 7, 40–47. [Google Scholar] [CrossRef]
- van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Zembruski, N.C.; Stache, V.; Haefeli, W.E.; Weiss, J. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal. Biochem. 2012, 429, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Taninaka, A.; Shigekawa, H.; Matsui, H. The cytotoxicity of cyclophosphamide is enhanced in combination with monascus pigment. J. Clin. Biochem. Nutr. 2021, 69, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Negrette-Guzmán, M. Combinations of the antioxidants sulforaphane or curcumin and the conventional antineoplastics cisplatin or doxorubicin as prospects for anticancer chemotherapy. Eur. J. Pharmacol. 2019, 859, 172513. [Google Scholar] [CrossRef]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity-Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Forment, J.V.; O’Connor, M.J. Targeting the replication stress response in cancer. Pharmacol. Ther. 2018, 188, 155–167. [Google Scholar] [CrossRef]
- Gibadullina, E.; Nguyen, T.T.; Strelnik, A.; Sapunova, A.; Voloshina, A.; Sudakov, I.; Vyshtakalyuk, A.; Voronina, J.; Pudovik, M.; Burilov, A. New 2,6-diaminopyridines containing a sterically hindered benzylphosphonate moiety in the aromatic core as potential antioxidant and anti-cancer drugs. Eur. J. Med. Chem. 2019, 184, 111735. [Google Scholar] [CrossRef]
- Dinesha; Viveka, S.; Priya, B.K.; Pai, K.S.; Naveen, S.; Lokanath, N.K.; Nagaraja, G.K. Synthesis and pharmacological evaluation of some new fluorine containing hydroxypyrazolines as potential anticancer and antioxidant agents. Eur. J. Med. Chem. 2015, 104, 25–32. [Google Scholar] [CrossRef]
- Kálai, T.; Kuppusamy, M.L.; Balog, M.; Selvendiran, K.; Rivera, B.K.; Kuppusamy, P.; Hideg, K. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J. Med. Chem. 2011, 54, 5414–5421. [Google Scholar] [CrossRef] [PubMed]
- Etxebeste-Mitxeltorena, M.; Plano, D.; Astrain-Redín, N.; Morán-Serradilla, C.; Aydillo, C.; Encío, I.; Moreno, E.; Espuelas, S.; Sanmartín, C. New Amides and Phosphoramidates Containing Selenium: Studies on Their Cytotoxicity and Antioxidant Activities in Breast Cancer. Antioxidants 2021, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.; Mariano, D.O.; Nedel, F.; Schultze, E.; Campos, V.F.; Seixas, F.; da Silva, R.S.; Munchen, T.S.; Ilha, V.; Dornelles, L.; et al. New organochalcogen multitarget drug: Synthesis and antioxidant and antitumoral activities of chalcogenozidovudine derivatives. J. Med. Chem. 2015, 58, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Martín, G.; Plano, D.; Encío, I.; Sanmartín, C. Novel N,N’-Disubstituted Selenoureas as Potential Antioxidant and Cytotoxic Agents. Antioxidants 2021, 10, 777. [Google Scholar] [CrossRef]










| Cell Lines | |||||
|---|---|---|---|---|---|
| Compounds | Breast | Prostate | Lung | Lung | |
| MDA-MB-231 | DU-145 | HTB-54 | BEAS-2B | SI a | |
| 1.I | 8.8 ± 4.8 | 9.5 ± 2.5 | 7.8 ± 12.7 | >100 | >12.7 |
| 2.I | n.d b | n.d b | 8.6 ± 1.1 | 5.3 ± 0.6 | 0.6 |
| 5.I | 15.5 ± 6.3 | 11.5 ± 3.4 | 8.4 ± 3.9 | >100 | >11.9 |
| 7.II | n.d b | n.d b | 12.0 ± 2.7 | 6.3 ± 1.5 | 0.5 |
| 10.II | n.d b | n.d b | 43. 0 ± 4.1 | 29.9 ± 5.0 | 0.7 |
| 2.III | n.d b | n.d b | 11.6 ± 1.6 | 26.6 ± 4.0 | 2.3 |
| 8.III | n.d b | n.d b | 21.7 ± 4.1 | >100 | >4.6 |
| Cisplatin | 5.5 ± 0.5 | 6.2 ± 0.1 | 13.68 [32] | 8.63 [33] | 0.63 |
| DPPH Inhibitory% | ||||
|---|---|---|---|---|
| R1 | Allyl Chain | Propargyl Chain | ||
 |  |  |  | |
| Selenoester a | Acylselenourea | Selenoester a | Acylselenourea | |
| phenyl | 9.0 ± 1.2 | 97.4 ± 0.8 | 3.5 ± 0.9 | 54.6 ± 0.8 |
| 5-benzo[d][1,3]dioxyl | 0.0 ± 2.3 | 88.2 ± 1.3 | 4.2 ± 8.0 | 62.4 ± 3.1 |
| 2-phenylvinyl | 3.6 ± 5.3 | 26.7 ± 0.6 | 2.4 ± 2.0 | |
| 3-pyridyl | 6.3 ± 2.9 | 96.2 ± 0.2 | 7.4 ± 0.7 | |
| 2-thiophenyl | 7.8 ± 10.4 | 97.3 ± 0.8 | 0.0 ± 2.1 | 33.0 ± 3.1 |
| 2-furyl | 4.6 ± 7.5 | 96.6 ± 0.6 | 5.4 ± 2.3 | 42.6 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astrain-Redin, N.; Raza, A.; Encío, I.; Sharma, A.K.; Plano, D.; Sanmartín, C. Novel Acylselenourea Derivatives: Dual Molecules with Anticancer and Radical Scavenging Activity. Antioxidants 2023, 12, 1331. https://doi.org/10.3390/antiox12071331
Astrain-Redin N, Raza A, Encío I, Sharma AK, Plano D, Sanmartín C. Novel Acylselenourea Derivatives: Dual Molecules with Anticancer and Radical Scavenging Activity. Antioxidants. 2023; 12(7):1331. https://doi.org/10.3390/antiox12071331
Chicago/Turabian StyleAstrain-Redin, Nora, Asif Raza, Ignacio Encío, Arun K. Sharma, Daniel Plano, and Carmen Sanmartín. 2023. "Novel Acylselenourea Derivatives: Dual Molecules with Anticancer and Radical Scavenging Activity" Antioxidants 12, no. 7: 1331. https://doi.org/10.3390/antiox12071331
APA StyleAstrain-Redin, N., Raza, A., Encío, I., Sharma, A. K., Plano, D., & Sanmartín, C. (2023). Novel Acylselenourea Derivatives: Dual Molecules with Anticancer and Radical Scavenging Activity. Antioxidants, 12(7), 1331. https://doi.org/10.3390/antiox12071331