The Effects of Lipoic Acid on Yolk Nutrient Utilization, Energy Metabolism, and Redox Balance over Time in Artemia sp.
Abstract
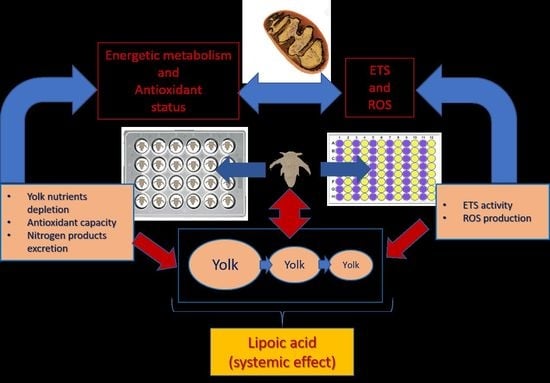
:1. Introduction
2. Materials and Methods
2.1. Artemia sp.
2.2. Standardization of the In Vivo Electron Transport System (ETS) Activity Protocol
2.2.1. Isolation of the Mitochondrial Fraction
2.2.2. Measurement of the Reductive Capacity of Artemia Mitochondria
2.3. In Vivo Exposure of Artemia to Lipoic Acid (LA)
2.3.1. Experiment 1
2.3.2. Experiment 2
2.4. Biochemical Analysis
2.4.1. Sample Processing
2.4.2. Determination of Protein Concentration
2.4.3. Determination of Glucose Concentration
2.4.4. Determination of Lactate Concentration
2.4.5. Determination of Triglyceride Concentration
2.4.6. Determination of Total Antioxidant Capacity
2.5. Statistical Analysis
3. Results
3.1. Standardization of Activity Protocol for the In Vivo Electron Transport System (ETS)
3.2. Measurement of the Reductive Capacity of Artemia Nauplii Mitochondria
3.3. Experiment 1
3.3.1. Protein Concentration
3.3.2. Total Ammoniacal Nitrogen (TAN)
3.3.3. Glucose Concentration
3.3.4. Lactate Concentration
3.3.5. Triglyceride Content
3.3.6. Total Antioxidant Capacity
3.3.7. PCA Analysis
3.4. Experiment 2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cristóbal-Azkarate, J.; Maréchal, L.; Semple, S.; Majolo, B.; MacLarnon, A. Metabolic Strategies in Wild Male Barbary Macaques: Evidence from Faecal Measurement of Thyroid Hormone. Biol. Lett. 2016, 12, 20160168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, T.; Texada, M.J.; Halberg, K.A.; Rewitz, K. Metabolism and Growth Adaptation to Environmental Conditions in Drosophila. Cell. Mol. Life Sci. 2020, 77, 4523–4551. [Google Scholar] [CrossRef] [PubMed]
- Seibel, B.A.; Drazen, J.C. The Rate of Metabolism in Marine Animals: Environmental Constraints, Ecological Demands and Energetic Opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 2061–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Li, X.; Zheng, S.; Wu, G. Amino Acids Are Major Energy Substrates for Tissues of Hybrid Striped Bass and Zebrafish. Amino Acids 2017, 49, 2053–2063. [Google Scholar] [CrossRef]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Nutrition and Functions of Amino Acids in Aquatic Crustaceans. In Amino Acids in Nutrition and Health: Amino Acids in the Nutrition of Companion, Zoo and Farm Animals; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 169–198. ISBN 978-3-030-54462-1. [Google Scholar]
- Navarro, I.; Gutiérrez, J. Chapter 17 Fasting and Starvation. In Metabolic Biochemistry; Hochachka, P.W., Mommsen, T.P., Eds.; Biochemistry and Molecular Biology of Fishes; Elsevier: Amsterdam, The Netherlands, 1995; Volume 4, pp. 393–434. [Google Scholar]
- Wang, X.; Li, E.; Chen, L. A Review of Carbohydrate Nutrition and Metabolism in Crustaceans. N. Am. J. Aquac. 2016, 78, 178–187. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose Metabolism in Fish: A Review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, M.; Ozorio, R.O.A.; Pourkazemi, M.; Bai, S.C. Effects of Dietary L-Carnitine Supplements on Growth and Body Composition in Beluga Sturgeon (Huso Huso) Juveniles. J. Appl. Ichthyol. 2008, 24, 646–649. [Google Scholar] [CrossRef]
- Shi, X.C.; Jin, A.; Sun, J.; Tian, J.J.; Ji, H.; Chen, L.Q.; Du, Z.Y. The Protein-Sparing Effect of α-Lipoic Acid in Juvenile Grass Carp, Ctenopharyngodon Idellus: Effects on Lipolysis, Fatty Acid β-Oxidation and Protein Synthesis. Br. J. Nutr. 2018, 120, 977–987. [Google Scholar] [CrossRef]
- Carbone, D.; Faggio, C. Importance of Prebiotics in Aquaculture as Immunostimulants. Effects on Immune System of Sparus Aurata and Dicentrarchus Labrax. Fish Shellfish Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef]
- Encarnação, P. Functional Feed Additives in Aquaculture Feeds. Aquafeed Formul. 2016, 217–237. [Google Scholar] [CrossRef]
- Fuchs, V.I.; Schmidt, J.; Slater, M.J.; Zentek, J.; Buck, B.H.; Steinhagen, D. The Effect of Supplementation with Polysaccharides, Nucleotides, Acidifiers and Bacillus Strains in Fish Meal and Soy Bean Based Diets on Growth Performance in Juvenile Turbot (Scophthalmus maximus). Aquaculture 2015, 437, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kütter, M.T.; Monserrat, J.M.; Primel, E.G.; Caldas, S.S.; Tesser, M.B. Effects of Dietary α-Lipoic Acid on Growth, Body Composition and Antioxidant Status in the Plata Pompano Trachinotus marginatus (Pisces, Carangidae). Aquaculture 2012, 368–369, 29–35. [Google Scholar] [CrossRef]
- Xu, N.; Fu, J.; Wang, H.; Lu, L. Quercetin Counteracts the Pro-Viral Effect of Heat Shock Response in Grass Carp Cells with Its Therapeutic Potential against Aquareovirus. Aquac. Res. 2021, 52, 3164–3173. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, R.; Qin, C.; Nie, G. Precision Nutritional Regulation and Aquaculture. Aquac. Rep. 2020, 18, 100496. [Google Scholar] [CrossRef]
- Lu, D.L.; Limbu, S.M.; Lv, H.B.; Ma, Q.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. The Comparisons in Protective Mechanisms and Efficiencies among Dietary α-Lipoic Acid, β-Glucan and l-Carnitine on Nile Tilapia Infected by Aeromonas Hydrophila. Fish Shellfish Immunol. 2019, 86, 785–793. [Google Scholar] [CrossRef]
- Panserat, S.; Skiba-Cassy, S.; Seiliez, I.; Lansard, M.; Plagnes-Juan, E.; Vachot, C.; Aguirre, P.; Larroquet, L.; Chavernac, G.; Medale, F.; et al. Metformin Improves Postprandial Glucose Homeostasis in Rainbow Trout Fed Dietary Carbohydrates: A Link with the Induction of Hepatic Lipogenic Capacities? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R707–R715. [Google Scholar] [CrossRef] [Green Version]
- Walsh, M.C.; Jacquier, V.; Schyns, G.; Claypool, J.; Tamburini, I.; Blokker, B.; Geremia, J.M. A Novel Microbiome Metabolic Modulator Improves the Growth Performance of Broiler Chickens in Multiple Trials and Modulates Targeted Energy and Amino Acid Metabolic Pathways in the Cecal Metagenome. Poult. Sci. 2021, 100, 100800. [Google Scholar] [CrossRef]
- Huang, C.-C.; Sun, J.; Ji, H.; Kaneko, G.; Xie, X.-D.; Chang, Z.-G.; Deng, W. Systemic Effect of Dietary Lipid Levels and α-Lipoic Acid Supplementation on Nutritional Metabolism in Zebrafish (Danio rerio): Focusing on the Transcriptional Level. Fish Physiol. Biochem. 2020, 46, 1631–1644. [Google Scholar] [CrossRef]
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic Acid as an Anti-Inflammatory and Neuroprotective Treatment for Alzheimer’s Disease. Adv. Drug Deliv. Rev. 2008, 60, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Sohaib, M.; Anjum, F.M.; Nasir, M.; Saeed, F.; Arshad, M.S.; Hussain, S. Alpha-Lipoic Acid: An Inimitable Feed Supplement for Poultry Nutrition. J. Anim. Physiol. Anim. Nutr. 2018, 102, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-L.; Kang, C.-H.; Wang, S.-G.; Lee, H.-M. α-Lipoic Acid Regulates Lipid Metabolism through Induction of Sirtuin 1 (SIRT1) and Activation of AMP-Activated Protein Kinase. Diabetologia 2012, 55, 1824–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Xiong, Y.; Zheng, J.; Zhou, D.; Kong, Y.; Qi, C.; Liu, Y.; Ye, J.; Limbu, S.M. Modulation of Growth, Antioxidant Status, Hepatopancreas Morphology, and Carbohydrate Metabolism Mediated by Alpha-Lipoic Acid in Juvenile Freshwater Prawns Macrobrachium nipponense under Two Dietary Carbohydrate Levels. Aquaculture 2022, 546, 737314. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Han, F.; Qi, C.; Li, E.; Guo, J.; Qin, J.G.; Chen, L. α-Lipoic Acid Regulate Growth, Antioxidant Status and Lipid Metabolism of Chinese Mitten Crab Eriocheir sinensis: Optimum Supplement Level and Metabonomics Response. Aquaculture 2019, 506, 94–103. [Google Scholar] [CrossRef]
- Terjesen, B.F.; Park, K.; Tesser, M.B.; Portella, M.C.; Zhang, Y.; Dabrowski, K. Biochemical and Molecular Actions of Nutrients Lipoic Acid and Ascorbic Acid Affect Plasma Free Amino Acids Selectively in the Teleost Fish Pacu (Piaractus mesopotamicus). J. Nutr. 2004, 134, 2930–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, M.C.; Massa, M.L.; Schinella, G.; Gagliardino, J.J.; Francini, F. Lipoic Acid Prevents Liver Metabolic Changes Induced by Administration of a Fructose-Rich Diet. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Lei, Y.; Xu, W.; Zhang, Y.; Zhou, H.; Zhang, W.; Mai, K. Protective Effects of Dietary α-Lipoic Acid on Abalone Haliotis Discus Hannai against the Oxidative Damage under Waterborne Cadmium Stress. Aquac. Nutr. 2019, 25, 263–270. [Google Scholar] [CrossRef]
- Tong, X.; Yang, X.; Bao, C.; Wang, J.; Tang, X.; Jiang, D.; Yang, L. Changes of Biochemical Compositions during Development of Eggs and Yolk-Sac Larvae of Turbot Scophthalmus maximus. Aquaculture 2017, 473, 317–326. [Google Scholar] [CrossRef]
- Vázquez, R.; González, S.; Rodríguez, A.; Mourente, G. Biochemical Composition and Fatty Acid Content of Fertilized Eggs, Yolk Sac Stage Larvae and First-Feeding Larvae of the Senegal Sole (Solea senegalensis Kaup). Aquaculture 1994, 119, 273–286. [Google Scholar] [CrossRef]
- Reid, R.M.; D’Aquila, A.L.; Biga, P.R. The Validation of a Sensitive, Non-Toxic in Vivo Metabolic Assay Applicable across Zebrafish Life Stages. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 208, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.V.; Maltez, L.C.; Ferreira, C.C.; Oliveira, T.P.A.P.; Sampaio, L.A.; Monserrat, J.M. ROS in Vivo Determination and Antioxidant Responses in Rotifers Brachionus plicatilis Fed with Commercial Yeast Saccharomyces cerevisiae or Microalgae Nannochloropsis oculata. Aquac. Int. 2021, 29, 1657–1667. [Google Scholar] [CrossRef]
- Rodriguez-Armenta, C.; Uribe-Carvajal, S.; Rosas-Lemus, M.; Chiquete-Felix, N.; Huerta-Ocampo, J.A.; Muhlia-Almazan, A. Alternative Mitochondrial Respiratory Chains from Two Crustaceans: Artemia franciscana Nauplii and the White Shrimp, Litopenaeus vannamei. J. Bioenerg. Biomembr. 2018, 50, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Grey, J.F.E.; Townley, A.R.; Everitt, N.M.; Campbell-Ritchie, A.; Wheatley, S.P. A Cost-Effective, Analytical Method for Measuring Metabolic Load of Mitochondria. Metab. Open 2019, 4, 100020. [Google Scholar] [CrossRef] [PubMed]
- Paffenhöfer, G.-A. Caloric Content of Larvae of the Brine Shrimp Artemia salina. Helgoländer Wiss. Meeresunters. 1967, 16, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Helder, W.; De Vries, R.T.P. An Automatic Phenol-Hypochlorite Method for the Determination of Ammonia in Sea- and Brackish Waters. Neth. J. Sea Res. 1979, 13, 154–160. [Google Scholar] [CrossRef]
- Krohn, R.I. The Colorimetric Detection and Quantitation of Total Protein. Curr. Protoc. Toxicol. 2005, 23, A.3I.1–A.3I.28. [Google Scholar] [CrossRef]
- Amado, L.L.; Garcia, M.L.; Ramos, P.B.; Freitas, R.F.; Zafalon, B.; Ferreira, J.L.R.; Yunes, J.S.; Monserrat, J.M. A Method to Measure Total Antioxidant Capacity against Peroxyl Radicals in Aquatic Organisms: Application to Evaluate Microcystins Toxicity. Sci. Total Environ. 2009, 407, 2115–2123. [Google Scholar] [CrossRef]
- Mendez-Romero, O.; Ricardez-García, C.; Castañeda-Tamez, P.; Chiquete-Félix, N.; Uribe-Carvajal, S. Thriving in Oxygen While Preventing ROS Overproduction: No Two Systems Are Created Equal. Front. Physiol. 2022, 13, 582. [Google Scholar] [CrossRef]
- Talbot, J.D.; Barrett, J.N.; Barrett, E.F.; David, G. Rapid, Stimulation-Induced Reduction of C12-Resorufin in Motor Nerve Terminals: Linkage to Mitochondrial Metabolism. J. Neurochem. 2008, 105, 807–819. [Google Scholar] [CrossRef]
- Springer, J.E.; Azbill, R.D.; Carlson, S.L. A Rapid and Sensitive Assay for Measuring Mitochondrial Metabolic Activity in Isolated Neural Tissue. Brain Res. Protoc. 1998, 2, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Evjemo, J.O.; Danielsen, T.L.; Olsen, Y. Losses of Lipid, Protein and N−3 Fatty Acids in Enriched Artemia Franciscana Starved at Different Temperatures. Aquaculture 2001, 193, 65–80. [Google Scholar] [CrossRef]
- Helland, S.; Triantaphyllidis, G.V.; Fyhn, H.J.; Evjen, M.S.; Lavens, P.; Sorgeloos, P. Modulation of the Free Amino Acid Pool and Protein Content in Populations of the Brine Shrimp Artemia spp. Mar. Biol. 2000, 137, 1005–1016. [Google Scholar] [CrossRef]
- Tanaka, K. The Proteasome: From Basic Mechanisms to Emerging Roles. Keio J. Med. 2013, 62, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Warner, A.H.; Puodziukas, J.G.; Finamore, F.J. Yolk Platelets in Brine Shrimp Embryos: Site of Biosynthesis and Storage of the Diguanosine Nucleotides. Exp. Cell Res. 1972, 70, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.H.; Perz, M.J.; Osahan, J.K.; Zielinski, B.S. Potential Role in Development of the Major Cysteine Protease in Larvae of the Brine Shrimp Artemia franciscana. Cell Tissue Res. 1995, 282, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Feng, L.; Tang, L.; Liu, Y.; Jiang, W.; Zhou, X. Growth Rate, Body Composition, Digestive Enzymes and Transaminase Activities, and Plasma Ammonia Concentration of Different Weight Jian Carp (Cyprinus Carpio Var. Jian). Anim. Nutr. 2015, 1, 373–377. [Google Scholar] [CrossRef]
- Herbeck, L.S.; Krumme, U.; Nordhaus, I.; Jennerjahn, T.C. Pond Aquaculture Effluents Feed an Anthropogenic Nitrogen Loop in a SE Asian Estuary. Sci. Total Environ. 2021, 756, 144083. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.C.; Amat, F.; Sargent, J.R. The Lipids of the Cysts of Freshwater- and Marine-Type Artemia. Aquaculture 1993, 109, 327–336. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Julia Xu, X.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A Long-Standing Partnership? Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, E.; Liu, S.; Huang, Z.; Qin, J.G.; Chen, L. Effects of α-Lipoic Acid on Growth Performance, Body Composition, Antioxidant Status and Lipid Catabolism of Juvenile Chinese Mitten Crab Eriocheir Sinensis Fed Different Lipid Percentage. Aquaculture 2018, 484, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Clegg, J.S. The Origin of Threhalose and Its Significance during the Formation of Encysted Dormant Embryos of Artemia salina. Comp. Biochem. Physiol. 1965, 14, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.P.; Huggins, A.K. Biochemical Changes Occurring during Morphogenesis of the Brine Shrimp Artemia Salina and the Effect of Alterations in Salinity. Comp. Biochem. Physiol. Part A Physiol. 1977, 57, 17–22. [Google Scholar] [CrossRef]
- Vallejo, C.G. Artemia Trehalase: Regulation by Factors That Also Control Resumption of Development. In Cell and Molecular Biology of Artemia Development; Warner, A.H., MacRae, T.H., Bagshaw, J.C., Eds.; Springer: Boston, MA, USA, 1989; pp. 173–189. ISBN 978-1-4757-0004-6. [Google Scholar]
- Xiong, Y.; Li, Q.; Ding, Z.; Zheng, J.; Zhou, D.; Wei, S.; Han, X.; Cheng, X.; Li, X.; Xue, Y. Dietary α-Lipoic Acid Requirement and Its Effects on Antioxidant Status, Carbohydrate Metabolism, and Intestinal Microflora in Oriental River Prawn Macrobrachium Nipponense (De Haan). Aquaculture 2022, 547, 737531. [Google Scholar] [CrossRef]
- Sokolova, I. Bioenergetics in Environmental Adaptation and Stress Tolerance of Aquatic Ectotherms: Linking Physiology and Ecology in a Multi-Stressor Landscape. J. Exp. Biol. 2021, 224, jeb236802. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, A.; Tretter, L.; Adam-Vizi, V. Inhibition of the Alpha-Ketoglutarate Dehydrogenase-Mediated Reactive Oxygen Species Generation by Lipoic Acid. J. Neurochem. 2009, 109, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. Emerging Therapeutic Roles for NAD+ Metabolism in Mitochondrial and Age-Related Disorders. Clin. Transl. Med. 2016, 5, e25. [Google Scholar] [CrossRef] [Green Version]
- Corona, J.C.; Duchen, M.R. PPARγ as a Therapeutic Target to Rescue Mitochondrial Function in Neurological Disease. Free Radic. Biol. Med. 2016, 100, 153. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Metcalfe, N.B.; Salin, K. Is Mitochondrial Reactive Oxygen Species Production Proportional to Oxygen Consumption? A Theoretical Consideration. BioEssays 2021, 43, 2000165. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Zeng, L.; Shen, B.; Xu, M.-Y.; Zhu, A.-Y.; Wu, C.-W. Antioxidant Defenses at Transcriptional and Enzymatic Levels and Gene Expression of Nrf2-Keap1 Signaling Molecules in Response to Acute Zinc Exposure in the Spleen of the Large Yellow Croaker Pseudosciaena crocea. Fish Shellfish Immunol. 2016, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.R.; Imai, S. The Dynamic Regulation of NAD Metabolism in Mitochondria. Trends Endocrinol. Metab. 2012, 23, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moini, H.; Packer, L.; Saris, N.-E.L. Antioxidant and Prooxidant Activities of α-Lipoic Acid and Dihydrolipoic Acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolzan, L.P.; Barroso, D.C.; Souza, C.F.; Oliveira, F.C.; Wagner, R.; Baldisserotto, B.; Val, A.L.; Baldissera, M.D. Dietary Supplementation with Nerolidol Improves the Antioxidant Capacity and Muscle Fatty Acid Profile of Brycon Amazonicus Exposed to Acute Heat Stress. J. Therm. Biol. 2021, 99, 103003. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK Facilitates Nuclear Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buitrago Ramírez, J.R.; Marreiro Gomes, R.M.; de Sousa Araujo, A.C.; Muñoz Buitrago, S.A.; Piraine Souza, J.; Monserrat, J.M. The Effects of Lipoic Acid on Yolk Nutrient Utilization, Energy Metabolism, and Redox Balance over Time in Artemia sp. Antioxidants 2023, 12, 1439. https://doi.org/10.3390/antiox12071439
Buitrago Ramírez JR, Marreiro Gomes RM, de Sousa Araujo AC, Muñoz Buitrago SA, Piraine Souza J, Monserrat JM. The Effects of Lipoic Acid on Yolk Nutrient Utilization, Energy Metabolism, and Redox Balance over Time in Artemia sp. Antioxidants. 2023; 12(7):1439. https://doi.org/10.3390/antiox12071439
Chicago/Turabian StyleBuitrago Ramírez, Juan Rafael, Robson Matheus Marreiro Gomes, Alan Carvalho de Sousa Araujo, Sonia Astrid Muñoz Buitrago, Jean Piraine Souza, and José María Monserrat. 2023. "The Effects of Lipoic Acid on Yolk Nutrient Utilization, Energy Metabolism, and Redox Balance over Time in Artemia sp." Antioxidants 12, no. 7: 1439. https://doi.org/10.3390/antiox12071439
APA StyleBuitrago Ramírez, J. R., Marreiro Gomes, R. M., de Sousa Araujo, A. C., Muñoz Buitrago, S. A., Piraine Souza, J., & Monserrat, J. M. (2023). The Effects of Lipoic Acid on Yolk Nutrient Utilization, Energy Metabolism, and Redox Balance over Time in Artemia sp. Antioxidants, 12(7), 1439. https://doi.org/10.3390/antiox12071439