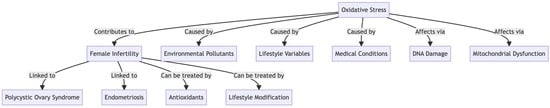
The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress
Abstract
:1. Introduction
2. ROS in the Female Reproductive System
2.1. Source of ROS Production in the Female Reproductive System
2.2. The Physiological Role of OS in Female Reproduction
2.3. Mechanisms by Which OS Damages the Female Reproductive System
2.4. What Are the Adverse Effects of OS on the Female Reproductive System?
2.4.1. Oocyte Quality
2.4.2. Embryonic Growth
2.4.3. Implantation
3. Factors Affecting Female Fertility Associated with OS
3.1. Age
3.2. Body Weight
3.3. Lifestyle Factors
3.4. Environmental and Occupational Exposures
4. OS Has Been Implicated in a Variety of Reproductive Disorders
4.1. Polycystic Ovarian Syndrome
4.2. Endometriosis
4.3. Unexplained Infertility
5. Pregnancy Complications
5.1. The Placenta
5.2. Spontaneous Abortion
5.3. Recurrent Pregnancy Loss
5.4. Preeclampsia
5.5. Intrauterine Growth Restriction
5.6. Preterm Labor
5.7. Ectopic Pregnancy
5.8. Gestational Diabetes
6. Possible Therapies to Treat the Harmful Effects of OS on the Female Reproductive System
6.1. Lifestyle Changes
6.2. Antioxidants
6.3. Medical Interventions
6.4. Assisted Reproductive Technology
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.P.; Roberts, V.H.; Myatt, L. Protein nitration in placenta—Functional significance. Placenta 2008, 29, 985–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Hennig, B.; Hammock, B.D.; Slim, R.; Toborek, M.; Saraswathi, V.; Robertson, L.W. PCB-induced oxidative stress in endothelial cells: Modulation by nutrients. Int. J. Hyg. Environ. Health 2002, 205, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Jirsova, S.; Masata, J.; Jech, L.; Zvarova, J. Effect of polychlorinated biphenyls (PCBs) and 1,1,1-trichloro-2,2,-bis(4-chlorophenyl)-ethane (DDT) in follicular fluid on the results of in vitro fertilization-embryo transfer (IVF-ET) programs. Fertil. Steril. 2010, 93, 1831–1836. [Google Scholar] [CrossRef]
- Samarawickrema, N.; Pathmeswaran, A.; Wickremasinghe, R.; Peiris-John, R.; Karunaratna, M.; Buckley, N.; Dawson, A.; de Silva, J. Fetal effects of environmental exposure of pregnant women to organophosphorus compounds in a rural farming community in Sri Lanka. Clin. Toxicol. 2008, 46, 489–495. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Roychoudhury, S. Reactive Oxygen Species in the Reproductive System: Sources and Physiological Roles. Adv. Exp. Med. Biol. 2022, 1358, 9–40. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [Green Version]
- Sugino, N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 2005, 4, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid. Redox Signal. 2020, 32, 522–535. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Luo, R.; Guo, X.; Li, R.; Ye, Z.; Peng, T.; Liu, F.; Yang, Z. Abnormal Endometrial Receptivity and Oxidative Stress in Polycystic Ovary Syndrome. Front. Pharmacol. 2022, 13, 904942. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, J.; Xu, J. Reactive oxygen and nitrogen species regulate porcine embryo development during pre-implantation period: A mini-review. Anim. Nutr. 2021, 7, 823–828. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Ray, S.D.; Lam, T.S.; Rotollo, J.A.; Phadke, S.; Patel, C.; Dontabhaktuni, A.; Mohammad, S.; Lee, H.; Strika, S.; Dobrogowska, A.; et al. Oxidative stress is the master operator of drug and chemically-induced programmed and unprogrammed cell death: Implications of natural antioxidants in vivo. Biofactors 2004, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, S.M.; Zachariae, R.; Mehlsen, M.Y.; Thomsen, D.; Hojgaard, A.; Ottosen, L.; Petersen, T.; Ingerslev, H.J. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: A prospective study. Hum. Reprod. 2009, 24, 2173–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free. Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician’s perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Alexander, R.W. Reactive oxygen species as mediators of angiogenesis signaling: Role of NAD(P)H oxidase. Mol. Cell. Biochem. 2004, 264, 85–97. [Google Scholar] [CrossRef]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef]
- Ra, K.; Park, S.C.; Lee, B.C. Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant. Int. J. Mol. Sci. 2023, 24, 5053. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S. Role of oxidative stress and protein oxidation in the aging process. Free. Radic. Biol. Med. 2002, 33, 37–44. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Rodriguez, A.; Gosalvez, J.; Agarwal, A.; Roy, R.; Johnston, S. DNA Damage and Repair in Human Reproductive Cells. Int. J. Mol. Sci. 2018, 20, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benko, F.; Ďuračka, M.; Baňas, Š.; Lukáč, N.; Tvrdá, E. Biological Relevance of Free Radicals in the Process of Physiological Capacitation and Cryocapacitation. Oxygen 2022, 2, 164–176. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [Green Version]
- Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology 2020, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boguenet, M.; Hachem, H.E.; Bouet, P.E.; Reynier, P. Embryo and Its Mitochondria. Antioxidants 2021, 10, 139. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, I.; Dhar, R.; Singh, S.; Sharma, J.B.; Nag, T.C.; Mridha, A.R.; Jaiswal, P.; Biswas, S.; Karmakar, S. Oxidative stress-induced impairment of trophoblast function causes preeclampsia through the unfolded protein response pathway. Sci. Rep. 2021, 11, 18415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, F. Effects of adverse fertility-related factors on mitochondrial DNA in the oocyte: A comprehensive review. Reprod. Biol. Endocrinol. 2023, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Crist, B.L.; Alekel, D.L.; Ritland, L.M.; Hanson, L.N.; Genschel, U.; Reddy, M.B. Association of oxidative stress, iron, and centralized fat mass in healthy postmenopausal women. J. Women’s Health 2009, 18, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffei, S.; Mercuri, A.; Prontera, C.; Zucchelli, G.C.; Vassalle, C. Vasoactive biomarkers and oxidative stress in healthy recently postmenopausal women treated with hormone replacement therapy. Climacteric 2006, 9, 452–458. [Google Scholar] [CrossRef]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef]
- Vincent, H.K.; Taylor, A.J. Biomarkers and Potential Mechanisms of Obesity-Induced Oxidant Stress in Humans. Int. J. Obes. 2005, 30, 400–418. [Google Scholar] [CrossRef] [Green Version]
- Brewer, C.T.; Balen, A.H. The Adverse Effects of Obesity on Conception and Implantation. Reproduction 2010, 140, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, S.; Murdoch, A. Obesity and Female Fertility: A Primary Care Perspective. J. Fam. Plan. Reprod. Health Care 2009, 35, 181–185. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-W.; Jia, X.; Qiao, J.; Guan, Y.; Kang, J. Adipokines in Reproductive Function: A Link between Obesity and Polycystic Ovary Syndrome. J. Mol. Endocrinol. 2013, 50, R21–R37. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Kimura, M.; Noma, K.; Sasaki, S.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; et al. Low body mass index is a risk factor for impaired endothelium-dependent vasodilation in humans: Role of nitric oxide and oxidative stress. J. Am. Coll. Cardiol. 2003, 42, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Okuka, M.; McLean, M.; Keefe, D.L.; Liu, L. Effects of cigarette smoke on fertilization and embryo development in vivo. Fertil. Steril. 2009, 92, 1456–1465. [Google Scholar] [CrossRef]
- Tiboni, G.M.; Bucciarelli, T.; Giampietro, F.; Sulpizio, M.; Di Ilio, C. Influence of cigarette smoking on vitamin E, vitamin A, beta-carotene and lycopene concentrations in human pre-ovulatory follicular fluid. Int. J. Immunopathol. Pharmacol. 2004, 17, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Savarese, B.; Coppola, L.; Navarra, G.; Avilia, G.; Laezza, C.; Bifulco, M. Cannabinoids in the Modulation of Oxidative Signaling. Int. J. Mol. Sci. 2023, 24, 2513. [Google Scholar] [CrossRef]
- Kovacic, P. Role of oxidative metabolites of cocaine in toxicity and addiction: Oxidative stress and electron transfer. Med. Hypotheses 2005, 64, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Fang, H.R.; Zhou, C.F.; Zhuang, Y.Y.; Zhang, P.; Gu, H.F.; Hu, B. A novel mechanism of formaldehyde neurotoxicity: Inhibition of hydrogen sulfide generation by promoting overproduction of nitric oxide. PLoS ONE 2013, 8, e54829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misner, M.J.; Taborek, A.; Dufour, J.; Sharifi, L.; Khokhar, J.Y.; Favetta, L.A. Effects of Delta-9 Tetrahydrocannabinol (THC) on Oocyte Competence and Early Embryonic Development. Front. Toxicol. 2021, 3, 14. [Google Scholar] [CrossRef]
- Petrelli, G.; Figa-Talamanca, I.; Lauria, L.; Mantovani, A. Spontaneous abortion in spouses of greenhouse workers exposed to pesticides. Environ. Health Prev. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef]
- Hennig, B.; Slim, R.; Toborek, M.; Robertson, L.W. Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells. J. Biochem. Mol. Toxicol. 1999, 13, 83–91. [Google Scholar] [CrossRef]
- Banerjee, B.D.; Seth, V.; Ahmed, R.S. Pesticide-induced oxidative stress: Perspectives and trends. Rev. Environ. Health 2001, 16, 1–40. [Google Scholar] [CrossRef]
- Jeng, H.A. Exposure to endocrine disrupting chemicals and male reproductive health. Front. Public Health 2014, 2, 55. [Google Scholar] [CrossRef] [Green Version]
- Caporossi, L.; Vigano, P.; Paci, E.; Capanna, S.; Alteri, A.; Campo, G.; Pigini, D.; De Rosa, M.; Tranfo, G.; Papaleo, B. Female Reproductive Health and Exposure to Phthalates and Bisphenol A: A Cross Sectional Study. Toxics 2021, 9, 299. [Google Scholar] [CrossRef]
- Rotterdam, E.A.-S.P.C.W.G. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxidative Med. Cell. Longev. 2016, 2016, 8589318. [Google Scholar] [CrossRef] [Green Version]
- Palacio, J.R.; Iborra, A.; Ulcova-Gallova, Z.; Badia, R.; Martinez, P. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin. Exp. Immunol. 2006, 144, 217–222. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; Banuls, C.; Alvarez, A.; de Pablo, C.; Sanchez-Serrano, M.; Gomez, M.; Hernandez-Mijares, A. Induction of oxidative stress and human leukocyte/endothelial cell interactions in polycystic ovary syndrome patients with insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, 3115–3122. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Carvalho, L.; Podgaec, S.; Bellodi-Privato, M.; Falcone, T.; Abrao, M.S. Role of eutopic endometrium in pelvic endometriosis. J. Minim. Invasive Gynecol. 2011, 18, 419–427. [Google Scholar] [CrossRef]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef] [Green Version]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Jackson, L.W.; Schisterman, E.F.; Dey-Rao, R.; Browne, R.; Armstrong, D. Oxidative stress and endometriosis. Hum. Reprod. 2005, 20, 2014–2020. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.A.; Santanam, N.; Parthasarathy, S. Endometriosis: A disease of oxidative stress? Semin. Reprod. Endocrinol. 1998, 16, 263–273. [Google Scholar] [CrossRef]
- Szczepanska, M.; Kozlik, J.; Skrzypczak, J.; Mikolajczyk, M. Oxidative stress may be a piece in the endometriosis puzzle. Fertil. Steril. 2003, 79, 1288–1293. [Google Scholar] [CrossRef]
- Mier-Cabrera, J.; Jimenez-Zamudio, L.; Garcia-Latorre, E.; Cruz-Orozco, O.; Hernandez-Guerrero, C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG 2011, 118, 6–16. [Google Scholar] [CrossRef]
- Sharma, I.; Dhaliwal, L.K.; Saha, S.C.; Sangwan, S.; Dhawan, V. Role of 8-iso-prostaglandin F2alpha and 25-hydroxycholesterol in the pathophysiology of endometriosis. Fertil. Steril. 2010, 94, 63–70. [Google Scholar] [CrossRef]
- Wang, Y.; Sharma, R.K.; Falcone, T.; Goldberg, J.; Agarwal, A. Importance of reactive oxygen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil. Steril. 1997, 68, 826–830. [Google Scholar] [CrossRef]
- Ho, H.N.; Wu, M.Y.; Chen, S.U.; Chao, K.H.; Chen, C.D.; Yang, Y.S. Total antioxidant status and nitric oxide do not increase in peritoneal fluids from women with endometriosis. Hum. Reprod. 1997, 12, 2810–2815. [Google Scholar] [CrossRef] [Green Version]
- Polak, G.; Koziol-Montewka, M.; Gogacz, M.; Blaszkowska, I.; Kotarski, J. Total antioxidant status of peritoneal fluid in infertile women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 94, 261–263. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Augoulea, A.; Mastorakos, G.; Lambrinoudaki, I.; Christodoulakos, G.; Creatsas, G. The role of the oxidative-stress in the endometriosis-related infertility. Gynecol. Endocrinol. 2009, 25, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Talbi, S.; Hamilton, A.E.; Vo, K.C.; Nyegaard, M.; Nezhat, C.R.; Lessey, B.A.; Giudice, L.C. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007, 148, 3814–3826. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.C.; Braga, P.C.; Martins, A.D.; Silva, B.M.; Alves, M.G.; Oliveira, P.F. Antioxidants Present in Reproductive Tract Fluids and Their Relevance for Fertility. Antioxidants 2021, 10, 1441. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2017, 79, e12782. [Google Scholar] [CrossRef]
- El-Kharoubi, A.F. Tubal Pathologies and Fertility Outcomes: A Review. Cureus 2023, 15, e38881. [Google Scholar] [CrossRef]
- Altmae, S.; Stavreus-Evers, A.; Ruiz, J.R.; Laanpere, M.; Syvanen, T.; Yngve, A.; Salumets, A.; Nilsson, T.K. Variations in folate pathway genes are associated with unexplained female infertility. Fertil. Steril. 2010, 94, 130–137. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.; Burton, G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am. J. Obstet. Gynecol. 2001, 184, 998–1003. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Lunghi, L.; Ferretti, M.E.; Medici, S.; Biondi, C.; Vesce, F. Control of human trophoblast function. Reprod. Biol. Endocrinol. 2007, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Sekizawa, A.; Purwosunu, Y.; Okazaki, S.; Farina, A.; Wibowo, N.; Shimizu, H.; Okai, T. Cellular mRNA expressions of anti-oxidant factors in the blood of preeclamptic women. Prenat. Diagn. 2009, 29, 691–696. [Google Scholar] [CrossRef]
- Hempstock, J.; Jauniaux, E.; Greenwold, N.; Burton, G.J. The contribution of placental oxidative stress to early pregnancy failure. Hum. Pathol. 2003, 34, 1265–1275. [Google Scholar] [CrossRef]
- Jauniaux, E.; Gulbis, B.; Burton, G.J. Physiological implications of the materno-fetal oxygen gradient in human early pregnancy. Reprod. Biomed. Online 2003, 7, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Nik, H.; Innes, B.; Lash, G.; Turner, M.; Drury, J.; Bulmer, J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum. Reprod. 2009, 24, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Hempstock, J.; Jauniaux, E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod. Biomed. Online 2003, 6, 84–96. [Google Scholar] [CrossRef]
- Gupta, S.; Agarwal, A.; Banerjee, J.; Alvarez, J.G. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obstet. Gynecol. Surv. 2007, 62, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Zachara, B.A.; Dobrzynski, W.; Trafikowska, U.; Szymanski, W. Blood selenium and glutathione peroxidases in miscarriage. BJOG 2001, 108, 244–247. [Google Scholar] [CrossRef]
- Al-Kunani, A.S.; Knight, R.; Haswell, S.J.; Thompson, J.W.; Lindow, S.W. The selenium status of women with a history of recurrent miscarriage. BJOG 2001, 108, 1094–1097. [Google Scholar] [CrossRef]
- Toy, H.; Camuzcuoglu, H.; Camuzcuoglu, A.; Celik, H.; Aksoy, N. Decreased serum prolidase activity and increased oxidative stress in early pregnancy loss. Gynecol. Obstet. Investig. 2010, 69, 122–127. [Google Scholar] [CrossRef]
- Toy, H.; Camuzcuoglu, H.; Celik, H.; Erel, O.; Aksoy, N. Assessment of serum paraoxonase and arylesterase activities in early pregnancy failure. Swiss Med. Wkly. 2009, 139, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, A.; Duley, L.; Crowther, C.; Haslam, R. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2005, CD004227. [Google Scholar] [CrossRef]
- Poston, L.; Raijmakers, M.T. Trophoblast oxidative stress, antioxidants and pregnancy outcome—A review. Placenta 2004, 25 (Suppl. A), S72–S78. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.M.; Farquharson, R.G. Predicting recurring miscarriage: What is important? Obstet. Gynecol. 1993, 82, 132–138. [Google Scholar]
- Rai, R.; Regan, L. Recurrent miscarriage. Lancet 2006, 368, 601–611. [Google Scholar] [CrossRef]
- Simsek, M.; Naziroglu, M.; Simsek, H.; Cay, M.; Aksakal, M.; Kumru, S. Blood plasma levels of lipoperoxides, glutathione peroxidase, beta carotene, vitamin A and E in women with habitual abortion. Cell Biochem. Funct. 1998, 16, 227–231. [Google Scholar] [CrossRef]
- Miller, H.; Wilson, R.; Jenkins, C.; MacLean, M.A.; Roberts, J.; Walker, J.J. Glutathione levels and miscarriage. Fertil. Steril. 2000, 74, 1257–1258. [Google Scholar] [CrossRef]
- Tempfer, C.; Unfried, G.; Zeillinger, R.; Hefler, L.; Nagele, F.; Huber, J.C. Endothelial nitric oxide synthase gene polymorphism in women with idiopathic recurrent miscarriage. Hum. Reprod. 2001, 16, 1644–1647. [Google Scholar] [CrossRef] [Green Version]
- Sata, F.; Yamada, H.; Kondo, T.; Gong, Y.; Tozaki, S.; Kobashi, G.; Kato, E.H.; Fujimoto, S.; Kishi, R. Glutathione S-transferase M1 and T1 polymorphisms and the risk of recurrent pregnancy loss. Mol. Hum. Reprod. 2003, 9, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Sata, F.; Yamada, H.; Yamada, A.; Kato, E.H.; Kataoka, S.; Saijo, Y.; Kondo, T.; Tamaki, J.; Minakami, H.; Kishi, R. A polymorphism in the CYP17 gene relates to the risk of recurrent pregnancy loss. Mol. Hum. Reprod. 2003, 9, 725–728. [Google Scholar] [CrossRef]
- Polimanti, R.; Piacentini, S.; Lazzarin, N.; Vaquero, E.; Re, M.A.; Manfellotto, D.; Fuciarelli, M. Glutathione S-transferase genes and the risk of recurrent miscarriage in Italian women. Fertil. Steril. 2012, 98, 396–400. [Google Scholar] [CrossRef]
- Polimanti, R.; Graziano, M.E.; Lazzarin, N.; Vaquero, E.; Manfellotto, D.; Fuciarelli, M. GSTO1 uncommon genetic variants are associated with recurrent miscarriage risk. Fertil. Steril. 2014, 101, 735–739. [Google Scholar] [CrossRef]
- Parveen, F.; Faridi, R.M.; Das, V.; Tripathi, G.; Agrawal, S. Genetic association of phase I and phase II detoxification genes with recurrent miscarriages among North Indian women. Mol. Hum. Reprod. 2010, 16, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.F.; Shaaban, O.M.; Bediawy, M.A. N-acetyl cysteine for treatment of recurrent unexplained pregnancy loss. Reprod. Biomed. Online 2008, 17, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, M.G.; De Flora, S.; D’Agostini, F.; Albini, A.; Fassina, G. Antiapoptotic and antigenotoxic effects of N-acetylcysteine in human cells of endothelial origin. Anticancer. Res. 2000, 20, 3183–3187. [Google Scholar]
- Cotgreave, I.A. N-acetylcysteine: Pharmacological considerations and experimental and clinical applications. Adv. Pharmacol. 1997, 38, 205–227. [Google Scholar] [PubMed]
- Kelly, G.S. Clinical applications of N-acetylcysteine. Altern. Med. Rev. 1998, 3, 114–127. [Google Scholar] [PubMed]
- Gupta, S.; Aziz, N.; Sekhon, L.; Agarwal, R.; Mansour, G.; Li, J.; Agarwal, A. Lipid peroxidation and antioxidant status in preeclampsia: A systematic review. Obstet. Gynecol. Surv. 2009, 64, 750–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reslan, O.M.; Khalil, R.A. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc. Hematol. Agents Med. Chem. 2010, 8, 204–226. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009, 30 (Suppl. A), S43–S48. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Jauniaux, E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Granger, J.P.; Alexander, B.T.; Llinas, M.T.; Bennett, W.A.; Khalil, R.A. Pathophysiology of preeclampsia: Linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation 2002, 9, 147–160. [Google Scholar] [CrossRef]
- Hung, T.H.; Skepper, J.N.; Burton, G.J. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am. J. Pathol. 2001, 159, 1031–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Yung, H.W. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens. 2011, 1, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Plowden, T.C.; Novak, C.M.; Spong, C.Y. Disparities in Obstetrical Outcomes in ART Pregnancies Compared With Natural Conceptions. Semin. Reprod. Med. 2013, 31, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.N.; Heazell, A.E.; Crocker, I.P.; Mor, G. Placental apoptosis in health and disease. Am. J. Reprod. Immunol. 2010, 64, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Aris, A.; Benali, S.; Ouellet, A.; Moutquin, J.M.; Leblanc, S. Potential biomarkers of preeclampsia: Inverse correlation between hydrogen peroxide and nitric oxide early in maternal circulation and at term in placenta of women with preeclampsia. Placenta 2009, 30, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.B.; Sharma, A.; Bahadur, A.; Vimala, N.; Satyam, A.; Mittal, S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int. J. Gynecol. Obstet. 2006, 94, 23–27. [Google Scholar] [CrossRef]
- Haque, S.K.; Siddiqui, M.U.; Islam, N.; Moin, S. Erythrocyte markers of oxidative stress in higher age-group preeclamptic and normal pregnant mothers. Hypertens. Pregnancy 2010, 29, 69–81. [Google Scholar] [CrossRef]
- Padmini, E.; Lavanya, S.; Uthra, V. Preeclamptic placental stress and over expression of mitochondrial HSP70. Clin. Chem. Lab. Med. 2009, 47, 1073–1080. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Bhattacharjee, J.; Vaid, M.; Madan, T.; Trivedi, S.S.; Sarma, P.U. Inducible nitric oxide synthase (iNOS) gene polymorphism in pre-eclampsia: A pilot study in North India. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 477–482. [Google Scholar] [CrossRef]
- Vural, P. Nitric oxide/endothelin-1 in preeclampsia. Clin. Chim. Acta 2002, 317, 65–70. [Google Scholar] [CrossRef]
- Shaamash, A.H.; Elsnosy, E.D.; Makhlouf, A.M.; Zakhari, M.M.; Ibrahim, O.A.; HM, E.L.-d. Maternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsia. Int. J. Gynecol. Obstet. 2000, 68, 207–214. [Google Scholar] [CrossRef]
- Tsukimori, K.; Fukushima, K.; Tsushima, A.; Nakano, H. Generation of reactive oxygen species by neutrophils and endothelial cell injury in normal and preeclamptic pregnancies. Hypertension 2005, 46, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Homuth, V.; Fischer, T.; Lindschau, C.; Horstkamp, B.; Jupner, A.; Baur, E.; Nissen, E.; Vetter, K.; Neichel, D.; et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Investig. 1999, 103, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, M.T.; Peters, W.H.; Steegers, E.A.; Poston, L. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women. Placenta 2004, 25 (Suppl. A), S85–S89. [Google Scholar] [CrossRef]
- Upritchard, J.E.; Schuurman, C.R.; Wiersma, A.; Tijburg, L.B.; Coolen, S.A.; Rijken, P.J.; Wiseman, S.A. Spread supplemented with moderate doses of vitamin E and carotenoids reduces lipid peroxidation in healthy, nonsmoking adults. Am. J. Clin. Nutr. 2003, 78, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dechend, R.; Viedt, C.; Muller, D.N.; Ugele, B.; Brandes, R.P.; Wallukat, G.; Park, J.K.; Janke, J.; Barta, P.; Theuer, J.; et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 2003, 107, 1632–1639. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.M.; Klein, R.L.; Haeri, S.; Moss, K.L.; Boggess, K.A. Association of midgestational paraoxonase 1 activity with pregnancies complicated by preeclampsia. Am. J. Perinatol. 2010, 27, 205–210. [Google Scholar] [CrossRef]
- Kumru, S.; Aydin, S.; Gursu, M.F.; Ozcan, Z. Changes of serum paraoxonase (an HDL-cholesterol-associated lipophilic antioxidant) and arylesterase activities in severe preeclamptic women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 114, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.; Benian, A.; Madazli, R.; Topcuoglu, M.A.; Aydin, S.; Albayrak, M. Circulating oxidized low-density lipoprotein and paraoxonase activity in preeclampsia. Gynecol. Obstet. Investig. 2005, 60, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 223–232. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Tang, G.; Ness, R.B.; Harger, G.; Roberts, J.M. Periconceptional multivitamin use reduces the risk of preeclampsia. Am. J. Epidemiol. 2006, 164, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Catov, J.M.; Nohr, E.A.; Bodnar, L.M.; Knudson, V.K.; Olsen, S.F.; Olsen, J. Association of periconceptional multivitamin use with reduced risk of preeclampsia among normal-weight women in the Danish National Birth Cohort. Am. J. Epidemiol. 2009, 169, 1304–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klemmensen, A.; Tabor, A.; Osterdal, M.L.; Knudsen, V.K.; Halldorsson, T.I.; Mikkelsen, T.B.; Olsen, S.F. Intake of vitamin C and E in pregnancy and risk of pre-eclampsia: Prospective study among 57,346 women. BJOG 2009, 116, 964–974. [Google Scholar] [CrossRef]
- Poston, L.; Briley, A.L.; Seed, P.T.; Kelly, F.J.; Shennan, A.H.; Vitamins in Pre-eclampsia Trial, C. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet 2006, 367, 1145–1154. [Google Scholar] [CrossRef]
- Roberts, J.M.; Myatt, L.; Spong, C.Y.; Thom, E.A.; Hauth, J.C.; Leveno, K.J.; Pearson, G.D.; Wapner, R.J.; Varner, M.W.; Thorp, J.M., Jr.; et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N. Engl. J. Med. 2010, 362, 1282–1291. [Google Scholar] [CrossRef] [Green Version]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192. [Google Scholar] [CrossRef]
- Potdar, N.; Singh, R.; Mistry, V.; Evans, M.D.; Farmer, P.B.; Konje, J.C.; Cooke, M.S. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG 2009, 116, 637–642. [Google Scholar] [CrossRef]
- Hung, T.H.; Burton, G.J. Hypoxia and reoxygenation: A possible mechanism for placental oxidative stress in preeclampsia. Taiwan. J. Obstet. Gynecol. 2006, 45, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Levy, R.; Smith, S.D.; Chandler, K.; Sadovsky, Y.; Nelson, D.M. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am. J. Physiol. Cell Physiol. 2000, 278, C982–C988. [Google Scholar] [CrossRef]
- Levy, R.; Smith, S.D.; Yusuf, K.; Huettner, P.C.; Kraus, F.T.; Sadovsky, Y.; Nelson, D.M. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am. J. Obstet. Gynecol. 2002, 186, 1056–1061. [Google Scholar] [CrossRef]
- Heazell, A.E.; Lacey, H.A.; Jones, C.J.; Huppertz, B.; Baker, P.N.; Crocker, I.P. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta 2008, 29, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yung, H.W.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG 2006, 113 (Suppl. S3), 17–42. [Google Scholar] [CrossRef]
- Norman, J.E.; Bollapragada, S.; Yuan, M.; Nelson, S.M. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth 2007, 7 (Suppl. S1), S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, M.D.; Pathak, R.; Ahmed, T.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Banerjee, B.D. Association of glutathione S-transferase M1 and T1 gene polymorphisms and oxidative stress markers in preterm labor. Clin. Biochem. 2010, 43, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Suke, S.G.; Ahmed, T.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Sharma, C.S.; Makhijani, S.D.; Banerjee, B.D. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum. Exp. Toxicol. 2010, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Lee, K.H.; Yi, C.H.; Ha, E.H.; Christiani, D.C. Genetic susceptibility of term pregnant women to oxidative damage. Toxicol. Lett. 2002, 129, 255–262. [Google Scholar] [CrossRef]
- Frosali, S.; Di Simplicio, P.; Perrone, S.; Di Giuseppe, D.; Longini, M.; Tanganelli, D.; Buonocore, G. Glutathione recycling and antioxidant enzyme activities in erythrocytes of term and preterm newborns at birth. Biol. Neonate 2004, 85, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Romero, R.; Tarca, A.L.; Draghici, S.; Erez, O.; Chaiworapongsa, T.; Kim, Y.M.; Kim, S.K.; Vaisbuch, E.; Tromp, G. Mitochondrial manganese superoxide dismutase mRNA expression in human chorioamniotic membranes and its association with labor, inflammation, and infection. J. Matern. Fetal. Neonatal Med. 2009, 22, 1000–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keelan, J.A.; Marvin, K.W.; Sato, T.A.; Coleman, M.; McCowan, L.M.; Mitchell, M.D. Cytokine abundance in placental tissues: Evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am. J. Obstet. Gynecol. 1999, 181, 1530–1536. [Google Scholar] [CrossRef]
- Cinkaya, A.; Keskin, H.L.; Buyukkagnici, U.; Gungor, T.; Keskin, E.A.; Avsar, A.F.; Bilge, U. Maternal plasma total antioxidant status in preterm labor. J. Obstet. Gynaecol. Res. 2010, 36, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.M.; Haeri, S.; Klein, R.L.; Boggess, K. Association of midgestation paraoxonase 1 activity and pregnancies complicated by preterm birth. Am. J. Obstet. Gynecol. 2010, 203, 246.e1–246.e4. [Google Scholar] [CrossRef]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P.; Wijnen, H.; Vader, H.; Kooistra, L.; Pop, V. Maternal selenium status during early gestation and risk for preterm birth. CMAJ 2011, 183, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.E.; Hwang, S.J.; Kim, B.H.; Sanders, K.M.; Ward, S.M. Myosalpinx Contractions Are Essential for Egg Transport Along the Oviduct and Are Disrupted in Reproductive Tract Diseases. Adv. Exp. Med. Biol. 2019, 1124, 265–294. [Google Scholar] [CrossRef]
- Ozdemir, E.U.; Bahat, P.Y.; Selçuki, N.F.T.; Cakmak, K.; Cakmak, F.; Neselioglu, S.; Erel, O. Evaluation of Oxidative Stress in Ectopic Pregnancies. Acta Biomed. 2022, 93, e2022025. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, J. Tubal ectopic pregnancy. BMJ Clin. Evid. 2015, 2015. [Google Scholar]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Hauguel-de Mouzon, S.; Jawerbaum, A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Murthy, K.A.S.; Bhandiwada, A.; Chandan, S.L.; Gowda, S.L.; Sindhusree, G. Evaluation of Oxidative Stress and Proinflammatory Cytokines in Gestational Diabetes Mellitus and Their Correlation with Pregnancy Outcome. Indian J. Endocrinol. Metab. 2018, 22, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic beta Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bell, H.K.; Bloomer, R.J. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxidative Med. Cell. Longev. 2009, 2, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Metwally, M.; Li, T.C.; Ledger, W.L. The impact of obesity on female reproductive function. Obes. Rev. 2007, 8, 515–523. [Google Scholar] [CrossRef]
- Abenhaim, H.A.; Kinch, R.A.; Morin, L.; Benjamin, A.; Usher, R. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Arch. Gynecol. Obstet. 2007, 275, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.B.; Vickers, M.H.; Hampton, M.B.; Poynton, R.A.; Sloboda, D.M. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS ONE 2010, 5, e15558. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online 2004, 9, 338–347. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbeck’s Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef]
- Pierce, J.D.; Cackler, A.B.; Arnett, M.G. Why should you care about free radicals? RN 2004, 67, 38–42. [Google Scholar]
- Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.J.; Han, H.; Zhou, G.B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledee-Bataille, N.; Olivennes, F.; Lefaix, J.L.; Chaouat, G.; Frydman, R.; Delanian, S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum. Reprod. 2002, 17, 1249–1253. [Google Scholar] [CrossRef] [Green Version]
- Thomson, R.L.; Spedding, S.; Buckley, J.D. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin. Endocrinol. 2012, 77, 343–350. [Google Scholar] [CrossRef]
- Badawy, A.; State, O.; Abdelgawad, S. N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: A cross-over trial. Acta Obstet. Gynecol. Scand. 2007, 86, 218–222. [Google Scholar] [CrossRef]
- Rodriguez-Varela, C.; Labarta, E. Clinical Application of Antioxidants to Improve Human Oocyte Mitochondrial Function: A Review. Antioxidants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Budani, M.C.; Tiboni, G.M. Effects of Supplementation with Natural Antioxidants on Oocytes and Preimplantation Embryos. Antioxidants 2020, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Klepcová, Z.; Špaková, I.; Urdzík, P.; Štofilová, J.; Bertková, I.; Kľoc, M.; Rabajdová, M. The Importance of Natural Antioxidants in Female Reproduction. Antioxidants 2023, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, A.; Ota, E.; Hori, H.; Miyazaki, C.; Crowther, C.A. Vitamin E supplementation in pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD004069. [Google Scholar] [CrossRef]
- Iervolino, M.; Lepore, E.; Forte, G.; Lagana, A.S.; Buzzaccarini, G.; Unfer, V. Natural Molecules in the Management of Polycystic Ovary Syndrome (PCOS): An Analytical Review. Nutrients 2021, 13, 1677. [Google Scholar] [CrossRef]
- Modarres, S.Z.; Asemi, Z.; Heidar, Z. The effects of selenium supplementation on glycemic control, serum lipoproteins and biomarkers of oxidative stress in infertile women diagnosed with polycystic ovary syndrome undergoing in vitro fertilization: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2022, 51, 92–96. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, H.; Liu, Y.; Zhao, S.; Zhao, H. Applications of Melatonin in Female Reproduction in the Context of Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 6668365. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sikka, S. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol. 2006, 18, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Florou, P.; Anagnostis, P.; Theocharis, P.; Chourdakis, M.; Goulis, D.G. Does coenzyme Q(10) supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J. Assist. Reprod. Genet. 2020, 37, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.A.M.; Kadir, S.H.S.A.; Arshad, A.H.; Aziz, N.A.; Nasir, N.A.A.; Latip, N.A. Are Vitamin E Supplementation Beneficial for Female Gynaecology Health and Diseases? Molecules 2022, 27, 1896. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Zikopoulos, A.; Moustakli, E.; Zachariou, A.; Tsirka, G.; Tsiampali, C.; Palapela, N.; Sofikitis, N.; Dimitriadis, F. The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress. Antioxidants 2023, 12, 1490. https://doi.org/10.3390/antiox12081490
Kaltsas A, Zikopoulos A, Moustakli E, Zachariou A, Tsirka G, Tsiampali C, Palapela N, Sofikitis N, Dimitriadis F. The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress. Antioxidants. 2023; 12(8):1490. https://doi.org/10.3390/antiox12081490
Chicago/Turabian StyleKaltsas, Aris, Athanasios Zikopoulos, Efthalia Moustakli, Athanasios Zachariou, Georgia Tsirka, Chara Tsiampali, Natalia Palapela, Nikolaos Sofikitis, and Fotios Dimitriadis. 2023. "The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress" Antioxidants 12, no. 8: 1490. https://doi.org/10.3390/antiox12081490
APA StyleKaltsas, A., Zikopoulos, A., Moustakli, E., Zachariou, A., Tsirka, G., Tsiampali, C., Palapela, N., Sofikitis, N., & Dimitriadis, F. (2023). The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress. Antioxidants, 12(8), 1490. https://doi.org/10.3390/antiox12081490