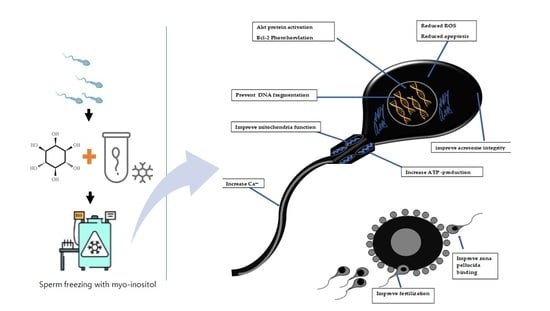
Antioxidant Effects of Myo-Inositol Improve the Function and Fertility of Cryopreserved Boar Semen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Semen Process, Extender Preparation, and Myo-Inositol Supplementation
2.3. Semen Cryopreservation and Thawing Procedure
2.4. Assessment of Semen Motility and Kinematics after the Freezing–Thawing Process
2.5. Assessment of Semen Viability after the Freezing–Thawing Process
2.6. Assessment of Acrosome Integrity after the Freezing–Thawing Process
2.7. Assessment of Caspase after the Freezing–Thawing Process
2.8. Assessment of Mitochondrial Membrane Integrity after the Freezing–Thawing Process
2.9. Assessment of Gene Expression after the Freezing–Thawing Process by One-Step Relative Quantitative PCR
2.10. Assessing In Vitro Fertilization Ability
2.10.1. Oocyte Maturation
2.10.2. In Vitro Fertilization
2.10.3. Embryo Culture
2.11. Assessment of Lipid Perodixation
2.12. Assessment of Sperm ROS after the Freezing–Thawing Process
2.13. Assessment of Sperm Apoptosis after the Freezing–Thawing Process
2.14. Experimental Design
2.15. Statistical Analysis
3. Results
3.1. Myo-Inositol Supplementation Effects on Frozen-Thawed Sperm Motility and Kinematics
3.2. Effects of Myo-Inositol Supplementation on Sperm Viability
3.3. Effects of Myo-Inositol Supplementation on the Integrity of Frozen-Thawed Sperm Acrosomes
3.4. Effects of Myo-Inositol Supplementation on Sperm Caspase
3.5. Effects of Myo-Inositol Supplementation on Sperm Mitochondrial Membrane Potential
3.6. Effects of Myo-Inositol Supplementation on Sperm Gene Expression
3.7. Effect of Myo-Inositol-Treated Cryopreserved Semen on Fertilization Ability and Blastocyst Formation
3.8. Effects of Myo-Inositol Supplementation on Sperm Lipid Peroxidation
3.9. Effect of Myo-Inositol on ROS Levels in Cryopreserved Semen
3.10. Effect of Myo-Inositol on Sperm Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinkar, D.; Sarkar, D.; Debbarma, A.; Rahman, M.; Swain, S.; Karunakaran, M. Review on recent advancement in semen additives for improving cryopreservation of bull semen. J. Entomol. 2020, 8, 1493–1505. [Google Scholar]
- Oldenhof, H.; Wolkers, W.F.; Sieme, H. Cryopreservation of Semen from Domestic Livestock: Bovine, Equine, and Porcine Sperm. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2021; pp. 365–377. [Google Scholar]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological Role of ROS in Sperm Function. In Male Infertility: Contemporary Clinical Approaches, Andrology, ART and Antioxidants; Parekattil, S.J., Esteves, S.C., Agarwal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 337–345. [Google Scholar]
- Kim, S.; Lee, Y.-J.; Kim, Y.-J. Changes in sperm membrane and ROS following cryopreservation of liquid boar semen stored at 15 °C. Anim. Reprod. Sci. 2011, 124, 118–124. [Google Scholar] [CrossRef]
- Sharafi, M.; Borghei-Rad, S.M.; Hezavehei, M.; Shahverdi, A.; Benson, J. Cryopreservation of Semen in Domestic Animals: A Review of Current Challenges, Applications, and Prospective Strategies. Animals 2022, 12, 3271. [Google Scholar] [CrossRef]
- Yeste, M.; Rodríguez-Gil, J.E.; Bonet, S. Artificial insemination with frozen-thawed boar sperm. Mol. Reprod. Dev. 2017, 84, 802–813. [Google Scholar] [CrossRef]
- da Silva, J.M. Artificial insemination and cryopreservation of boar semen: Current state and problematics. Open Sci. J. 2020, 5. [Google Scholar] [CrossRef]
- Techakumphu, M.; Buranaamnuay, K.; Tantasuparuk, W.; Am-In, N. Improvement of Semen Quality by Feed Supplement and Semen Cryopreservation in Swine; IntechOpen: London, UK, 2013. [Google Scholar]
- Pezo, F.; Yeste, M.; Zambrano, F.; Uribe, P.; Risopatrón, J.; Sánchez, R. Antioxidants and their effect on the oxidative/nitrosative stress of frozen-thawed boar sperm. Cryobiology 2021, 98, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Pezo, F.; Romero, F.; Zambrano, F.; Sanchez, R.S. Preservation of boar semen: An update. Reprod. Domest. Anim. 2019, 54, 423–434. [Google Scholar] [CrossRef]
- Jovicic, M.; Chmelikova, E.; Sedmikova, M. Cryopreservation of boar semen. Czech J. Anim. Sci. 2020, 65, 115–123. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Antioxidants in Sperm Cryopreservation. In Male Infertility; Springer International Publishing: Cham, Switzerland, 2020; pp. 671–678. [Google Scholar]
- Funahashi, H.; Sano, T. Select antioxidants improve the function of extended boar semen stored at 10 degrees C. Theriogenology 2005, 63, 1605–1616. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Heart 2022, 9, e001989. [Google Scholar] [CrossRef]
- Ponchia, R.; Bruno, A.; Renzi, A.; Landi, C.; Shaba, E.; Luongo, F.P.; Haxhiu, A.; Artini, P.G.; Luddi, A.; Governini, L.; et al. Oxidative Stress Measurement in Frozen/Thawed Human Sperm: The Protective Role of an In Vitro Treatment with Myo-Inositol. Antioxidants 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Malo, C.; Gil, L.; Gonzalez, N.; Martínez, F.; Cano, R.; de Blas, I.; Espinosa, E. Anti-oxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: Comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 2010, 61, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, P.; Wang, J.; Shi, T.; You, C. Production of myo-inositol: Recent advance and prospective. Biotechnol. Appl. Biochem. 2022, 69, 1101–1111. [Google Scholar] [CrossRef]
- De Luca, M.N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Holub, B.J. Metabolism and function of myo-inositol and inositol phospholipids. Annu. Rev. Nutr. 1986, 6, 563–597. [Google Scholar] [CrossRef]
- Dinkova, A.M.D.; Konova, E. Efficacy of myo-inositol in the clinical management of patients with asthenozoospermia. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 62–65. [Google Scholar] [PubMed]
- Kuşcu, N.; Bizzarri, M.; Bevilacqua, A. Myo-Inositol Safety in Pregnancy: From Preimplantation Development to Newborn Animals. Int. J. Endocrinol. 2016, 2016, 2413857. [Google Scholar] [CrossRef]
- Vazquez-Levin, M.H.; Verón, G.L. Myo-inositol in health and disease: Its impact on semen parameters and male fertility. Andrology 2020, 8, 277–298. [Google Scholar] [CrossRef]
- Chauvin, T.R.; Griswold, M.D. Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol. Reprod. 2004, 70, 744–751. [Google Scholar] [CrossRef]
- Saleh, R.; Assaf, H.; Abd El Maged, W.M.; Elsuity, M.; Fawzy, M. Increased cryo-survival rate in ejaculated human sperm from infertile men following pre-freeze in vitro myo-inositol supplementation. Clin. Exp. Reprod. Med. 2018, 45, 177–182. [Google Scholar] [CrossRef]
- Mohammadi, F.; Varanloo, N.; Heydari Nasrabadi, M.; Vatannejad, A.; Amjadi, F.S.; Javedani Masroor, M.; Bajelan, L.; Mehdizadeh, M.; Aflatoonian, R.; Zandieh, Z. Supplementation of sperm freezing medium with myoinositol improve human sperm parameters and protects it against DNA fragmentation and apoptosis. Cell Tissue Bank. 2019, 20, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Myoinositol Supplementation of Freezing Medium Improves the Quality-Related Parameters of Dog Sperm. Animals 2019, 9, 1038. [Google Scholar] [CrossRef]
- Pettitt, M.J.; Buhr, M.M. Extender components and surfactants affect boar sperm function and membrane behavior during cryopreservation. J. Androl. 1998, 19, 736–746. [Google Scholar]
- Areeg, A.; Rana, O.; Seongju, L.; Iljeoung, Y.; Yubyeol, J. Development of a new mini straw for cryopreservation of boar semen. J. Anim. Reprod. Biotechnol. 2022, 37, 113–120. [Google Scholar]
- Yu, I.J. Canine sperm cryopreservation using glucose in glycerol-free Tris. Cryo Lett. 2014, 35, 101–107. [Google Scholar]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Yu, I.-J. Dog Sperm Cryopreservation Using Glucose in Glycerol-free TRIS: Glucose Concentration, Exposure Time. J. Vet. Clin. 2013, 30, 442–448. [Google Scholar]
- Tatemoto, H.; Osokoshi, N.; Hirai, M.; Masuda, Y.; Konno, T.; Yamanaka, K. Addition of l-carnitine to the freezing extender improves post-thaw sperm quality of Okinawan native Agu pig. Theriogenology 2022, 188, 170–176. [Google Scholar] [CrossRef]
- Du, J.; Wang, Z.; Cui, Y.; Li, Y.; Yang, Y.; Li, P.; Liu, Y. Effects of salinity on Crassostrea angulata sperm quality and fertilization using PI/Rh123 dual fluorescent staining and flow cytometry. J. Fish. China 2018, 42, 1737–1746. [Google Scholar] [CrossRef]
- Bang, S.; Tanga, B.M.; Fang, X.; Seong, G.; Saadeldin, I.; Qamar, A.; Lee, S.; Kim, K.-J.; Park, Y.-J.; Talha, N.; et al. Cryopreservation of Pig Semen Using a Quercetin-Supplemented Freezing Extender. Life 2022, 12, 1155. [Google Scholar] [CrossRef]
- Almubarak, A.M.; Kim, E.; Yu, I.-J.; Jeon, Y. Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 2021, 169, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Cui, X.-S. Optimization of the in vitro fertilization system in pigs. J. Anim. Reprod. Biotechnol. 2023, 38, 70–76. [Google Scholar] [CrossRef]
- Abeydeera, L.R.; Wang, W.H.; Cantley, T.C.; Rieke, A.; Day, B.N. Coculture with follicular shell pieces can enhance the developmental competence of pig oocytes after in vitro fertilization: Relevance to intracellular glutathione. Biol. Reprod. 1998, 58, 213–218. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Determination of intracellular reactive oxygen species and high mitochondrial membrane potential in Percoll-treated viable boar sperm using fluorescence-activated flow cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Johannisson, A.; Wallgren, M.; Rodrıíguez-Martínez, H. Assessment of fresh and frozen–thawed boar semen using an Annexin-V assay: A new method of evaluating sperm membrane integrity. Theriogenology 2003, 60, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Casarosa, E.; Carletti, E.; Monteleone, P.; Di Noia, A.; Di Berardino, O.M. In vitro effect of myo-inositol on sperm motility in normal and oligoasthenospermia patients undergoing in vitro fertilization. Gynecol. Endocrinol. 2017, 33, 109–112. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Naveed, M.I.; Raza, S.; Fang, X.; Roy, P.K.; Bang, S.; Tanga, B.M.; Saadeldin, I.M.; Lee, S.; Cho, J. Role of antioxidants in fertility preservation of sperm—A narrative review. Anim. Biosci. 2023, 36, 385–403. [Google Scholar] [CrossRef]
- Bathgate, R. Antioxidant mechanisms and their benefit on post-thaw boar sperm quality. Reprod. Domest. Anim. 2011, 46 (Suppl. 2), 23–25. [Google Scholar] [CrossRef]
- Dong, R.; Luo, L.; Liu, X.; Yu, G. Effects of riboflavin on boar sperm motility, sperm quality, enzyme activity and antioxidant status during cryopreservation. Vet. Med. Sci. 2022, 8, 1509–1518. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, M.-K.; Song, H.-J.; Kang, E.-J.; Ock, S.-A.; Mohana Kumar, B.; Balasubramanian, S.; Rho, G.-J. Effect of α-tocopherol supplementation during boar semen cryopreservation on sperm characteristics and expression of apoptosis related genes. Cryobiology 2009, 58, 181–189. [Google Scholar] [CrossRef]
- de Mercado, E.; Tomás-Almenar, C.; Gómez-Izquierdo, E. Improvement of the motility of boar sperm after cryopreservation. Anim. Reprod. 2020, 222, 106610. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Papale, P.; Della Ragione, A.; Quaranta, G.; Russo, G.; Russo, S. In Vitro Antioxidant Treatment of Semen Samples in Assisted Reproductive Technology: Effects of Myo-Inositol on Nemaspermic Parameters. Int. J. Endocrinol. 2016, 2016, 2839041. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2017, 5, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, F.; Lobascio, A.M.; Terribile, M.; Casciani, V.; Greco, P.; Franco, G.; Minasi, M.G.; Greco, E. Analysis of MYO-Inositol effect on spermatozoa motility, in hyper viscous ejaculates and in patients with grades II and III varicocele. Arch. Ital. Urol. Androl. 2016, 88, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar]
- Liu, D.Y.; Clarke, G.N.; Baker, H.W. Hyper-osmotic condition enhances protein tyrosine phosphorylation and zona pellucida binding capacity of human sperm. Hum. Reprod. 2006, 21, 745–752. [Google Scholar] [CrossRef]
- Al-Mutary, M.G. Use of antioxidants to augment semen efficiency during liquid storage and cryopreservation in livestock animals: A review. J. King Saud Univ. -Sci. 2021, 33, 101226. [Google Scholar] [CrossRef]
- Valverde, A.; Madrigal, M.; Caldeira, C.; Bompart, D.; De Murga, J.N.; Arnau, S.; Soler, C. Effect of frame rate capture frequency on sperm kinematic parameters and subpopulation structure definition in boars, analysed with a CASA-Mot system. Reprod. Domest. Anim. 2019, 54, 167–175. [Google Scholar] [CrossRef]
- Montanino Oliva, M.; Buonomo, G.; Carra, M.C.; Lippa, A.; Lisi, F. Myo-inositol impact on sperm motility in vagina and evaluation of its effects on foetal development. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2704–2709. [Google Scholar] [CrossRef]
- Tanhaei Vash, N.; Nadri, P.; Karimi, A. Synergistic effects of myo-inositol and melatonin on cryopreservation of goat spermatozoa. Reprod. Domest. Anim. 2022, 57, 876–885. [Google Scholar] [CrossRef]
- Jawad, A.; Oh, D.; Choi, H.; Kim, M.; Cai, L.; Lee, J.; Hyun, S.-H. Myo-inositol improves the viability of boar sperm during liquid storage. Front. Vet. Sci. 2023, 10, 1150984. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Yeste, M.; Salas-Huetos, A. The Relationship between Sperm Oxidative Stress Alterations and IVF/ICSI Outcomes: A Systematic Review from Nonhuman Mammals. Biology 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.A.; Almiñana, C.; Roca, J.; Vázquez, J.M.; Martínez, E.A. Boar semen variability and its effects on IVF efficiency. Theriogenology 2008, 70, 1260–1268. [Google Scholar] [CrossRef]
- Knox, R.V. The Fertility of Frozen Boar Sperm When used for Artificial Insemination. Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 90–97. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Mongioì, L.M.; Vitale, S.G.; Laganà, A.S.; Cimino, L.; Calogero, A.E. Myo-inositol as a male fertility molecule: Speed them up! Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 30–35. [Google Scholar] [PubMed]
- Carlomagno, G.; Nordio, M.; Chiu, T.T.; Unfer, V. Contribution of myo-inositol and melatonin to human reproduction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 267–272. [Google Scholar] [CrossRef]
- Ghasemi, A.; Amjadi, F.; Masoumeh Ghazi Mirsaeed, S.; Mohammad Beigi, R.; Ghasemi, S.; Moradi, Y.; Tahereh Ghazi Mirsaeed, S. The effect of Myo-inositol on sperm parameters and pregnancy rate in oligoasthenospermic men treated with IUI: A randomized clinical trial. Int. J. Reprod. Biomed. 2019, 17, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Martecikova, S.; Hulinska, P.; Reckova, Z.; Pavlik, A.; Jeseta, M.; Machatkova, M. Effect of acrosome reaction progress in frozen-thawed boar spermatozoa on the efficiency of in vitro oocyte fertilization. Vet. Med. 2010, 55, 429–437. [Google Scholar] [CrossRef]
- Davies, R.; Jayasena, C.N.; Rai, R.; Minhas, S. The Role of Seminal Oxidative Stress in Recurrent Pregnancy Loss. Antioxidants 2023, 12, 723. [Google Scholar] [CrossRef]
- Lane, M.; McPherson, N.O.; Fullston, T.; Spillane, M.; Sandeman, L.; Kang, W.X.; Zander-Fox, D.L. Oxidative stress in mouse sperm impairs embryo development, fetal growth and alters adiposity and glucose regulation in female offspring. PLoS ONE 2014, 9, e100832. [Google Scholar] [CrossRef]
- Doğu, Z.; Şahinöz, E.; Aral, F.; Koyuncu, İ.; Yüksekdağ, Ö. Effects of Inositol Supplementation in Sperm Extender on the Quality of Cryopreserved Mesopotamian Catfish (Silurus triostegus, H. 1843) Sperm. Animals 2021, 11, 3029. [Google Scholar] [CrossRef]
- Kopeika, J.; Thornhill, A.; Khalaf, Y. The effect of cryopreservation on the genome of gametes and embryos: Principles of cryobiology and critical appraisal of the evidence. Hum. Reprod. Update 2015, 21, 209–227. [Google Scholar] [CrossRef]
- Abdolsamadi, M.; Mohammadi, F.; Nashtaei, M.S.; Teimouri, M.; Sardar, R.; Dayani, M.; Haghighi, M.; Ghasemi, S.; Vatannejad, A.; Zandieh, Z. Does myoinositol supplement improve sperm parameters and DNA integrity in patients with oligoasthenoteratozoospermia after the freezing-thawing process? Cell Tissue Bank. 2020, 21, 99–106. [Google Scholar] [CrossRef]
- Bucak, M.N.; Tuncer, P.B.; Sariozkan, S.; Baspinar, N.; Taspinar, M.; Coyan, K.; Bilgili, A.; Akalin, P.P.; Buyukleblebici, S.; Aydos, S.; et al. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: Antioxidants protect DNA integrity against cryodamage. Cryobiology 2010, 61, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Cheraghi, E.; Soleimani Mehranjani, M. Effect of Myo-inositol on sperm quality and biochemical factors in cryopreserved semen of patients with Asthenospermia. Andrologia 2022, 54, e14528. [Google Scholar] [CrossRef]
- Mohammadi, A.; Asadpour, R.; Tayefi-Nasrabadi, H.; Rahbar, M.; Joozani, R.J. Evaluation of Microscopic, Flow Cytometric, and Oxidative Parameters of the Frozen-Thawed Bull Sperm in a Freezing Extender Containing Myo-Inositol. Biopreserv. Biobank. 2022, 20, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Kulaksız, R.; Bucak, M.; Akcay, E.; Sakin, F.; Daskin, A.; Ateşşahin, A. The Effects of Different Extenders and Myo-Inositol on Post-thaw Quality of Ram Semen. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 217–222. [Google Scholar]





| Gene | Primer Sequence | Product Size (bp) | Accession Number |
|---|---|---|---|
| Bcl-2 | F:TGGTGGTTGACTTTCTCTCC R: ATTGATGGCACTAGGGGTTT | 752 | NM_214285.1 |
| BAX | F:CAGCTCTGAGCAGATCATGA R: TTGAGACACTCGCTCAACTT | 833 | XM_003127290.5 |
| GAPDH | F:CTCTGGCAAAGTGGACATTG R: CATTGATGACAAGCTTCCCG | 1341 | NM_001206359.1 |
| ROMO1 | F: GAAGATGGGCTTTGTGATGG R: ATAGTACATGGGCTGGGACT | 401 | NM_001097462.2 |
| SMOX | F: TGGAAGAGACAACTGATGGG R: CATGGTTATGGTCACCCTCA | 2034 | NM_001185170.1 |
| SMCP | F: AGTGCACCTGCCTGAATAAG R: CCTACTTGTTTGGCTGCTTC | 704 | NM_001008685.1 |
| NOX5 | F: TTCTTCGCCCTCTTTGACTT R: CAGTCAAAGTTGAGGCACTG | 8874 | XM_021100544.1 |
| Kinematic | Control | 0.5 mg/mL | 1 mg/mL | 1.5 mg/mL | 2 mg/mL |
|---|---|---|---|---|---|
| VLC | 59.2 ± 5.98 a | 54.53 ± 1.8 ab | 50.5 ± 1.4 aab | 48.4 ± 4.7 ab | 43.35 ± 1.9 b |
| VAP | 48.2 ± 3.11 a | 43.56 ± 1.18 ab | 39.81 ± 1.88 bc | 39.4 ± 3.47 bc | 35.3 ± 1.52 c |
| VSL | 40.5 ± 2.27 a | 35.26 ± 0.96 ab | 31.85 ± 1.45 b | 33.18 ± 3.62 ab | 29.91 ± 2.21 b |
| STR | 72.4 ± 4.74 a | 70.83 ± 0.70 a | 70.85 ± 2.80 a | 74.57 ± 1.35 a | 72.25 ± 3.51 a |
| LIN | 59.0 ± 5.45 a | 56.28 ± 0.72 a | 56.63 ± 2.47 a | 60.95 ± 1.53 a | 58.38 ± 2.63 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, R.; Lee, S.; Almubarak, A.; Han, J.-I.; Yu, I.-J.; Jeon, Y. Antioxidant Effects of Myo-Inositol Improve the Function and Fertility of Cryopreserved Boar Semen. Antioxidants 2023, 12, 1673. https://doi.org/10.3390/antiox12091673
Osman R, Lee S, Almubarak A, Han J-I, Yu I-J, Jeon Y. Antioxidant Effects of Myo-Inositol Improve the Function and Fertility of Cryopreserved Boar Semen. Antioxidants. 2023; 12(9):1673. https://doi.org/10.3390/antiox12091673
Chicago/Turabian StyleOsman, Rana, Seongju Lee, Areeg Almubarak, Jae-Ik Han, Il-Jeoung Yu, and Yubyeol Jeon. 2023. "Antioxidant Effects of Myo-Inositol Improve the Function and Fertility of Cryopreserved Boar Semen" Antioxidants 12, no. 9: 1673. https://doi.org/10.3390/antiox12091673
APA StyleOsman, R., Lee, S., Almubarak, A., Han, J. -I., Yu, I. -J., & Jeon, Y. (2023). Antioxidant Effects of Myo-Inositol Improve the Function and Fertility of Cryopreserved Boar Semen. Antioxidants, 12(9), 1673. https://doi.org/10.3390/antiox12091673