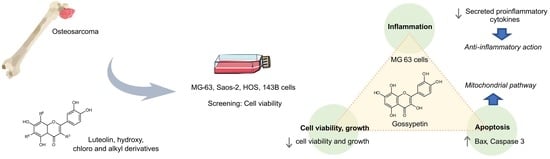
Gossypetin Is a Novel Modulator of Inflammatory Cytokine Production and a Suppressor of Osteosarcoma Cell Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Media and Reagents
2.2. Cell Culture and Exposure Conditions
2.3. Cytotoxicity Assays
2.4. Apoptosis Detection
2.5. Western Blot Analysis
2.6. Caspase Activity
2.7. Cytokine Production
2.8. Statistical Analysis
3. Results
3.1. Structure-Activity Relationship (SAR)
3.2. Gossypetin Induces the Intrinsic Apoptotic Pathway
3.3. Under Proinflammatory Conditions, Gossypetin Alters Cytokine Production in OS Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif. Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef]
- Gill, J.; Ahluwalia, M.K.; Geller, D.; Gorlick, R. New targets and approaches in osteosarcoma. Pharmacol. Ther. 2013, 137, 89–99. [Google Scholar] [CrossRef]
- Saraf, A.J.; Fenger, J.M.; Roberts, R.D. Osteosarcoma: Accelerating Progress Makes for a Hopeful Future. Front. Oncol. 2018, 8, 4. [Google Scholar] [CrossRef]
- Argenziano, M.; Tortora, C.; Pota, E.; Di Paola, A.; Di Martino, M.; Di Leva, C.; Di Pinto, D.; Rossi, F. Osteosarcoma in Children: Not Only Chemotherapy. Pharmaceuticals 2021, 14, 923. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Liu, J.B.; Wang, X.F.; Ma, Y.S.; Fu, D. Current Status and Prospects of Clinical Treatment of Osteosarcoma. Technol. Cancer Res. Treat. 2022, 21, 15330338221124696. [Google Scholar] [CrossRef]
- Long, J.S.; Ryan, K.M. New frontiers in promoting tumour cell death: Targeting apoptosis, necroptosis and autophagy. Oncogene 2012, 31, 5045–5060. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Li, Y.; Xia, J.; Li, D.; Li, H.; Ren, M.; Liao, Y.; Yu, S.; Chen, Y.; et al. Cell apoptosis, autophagy and necroptosis in osteosarcoma. Oncotarget 2016, 7, 44763. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 151654. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Magagnoli, F.; Luppi, S.; Serra, M. An update on emerging drugs in osteosarcoma: Towards tailored therapies? Expert Opin. Emerg. Drugs 2019, 24, 153–171. [Google Scholar] [CrossRef]
- Harris, M.A.; Hawkins, C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022, 23, 3817. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Cascini, C.; Chiodoni, C. The Immune Landscape of Osteosarcoma: Implications for Prognosis and Treatment Response. Cells 2021, 10, 1668. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, L.; Luo, G.; Son, H.; Prectoni, J.H.; Zheng, W. Effect of the cytokine levels in serum on osteosarcoma. Tumour Biol. 2014, 35, 1023–1028. [Google Scholar] [CrossRef]
- Gross, A.C.; Cam, H.; Phelps, D.A.; Saraf, A.J.; Bid, H.K.; Cam, M.; London, C.A.; Winget, S.A.; Arnold, M.A.; Brandolini, L.; et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight 2018, 3, e99791. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Du, L.; Fan, Q.M.; Tang, Z.; Tang, T.T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012, 325, 80–88. [Google Scholar] [CrossRef]
- Kawano, M.; Tanaka, K.; Itonaga, I.; Iwasaki, T.; Tsumura, H. Interaction between human osteosarcoma and mesenchymal stem cells via an interleukin-8 signaling loop in the tumor microenvironment. Cell Commun. Signal. 2018, 16, 13. [Google Scholar] [CrossRef]
- Yati, S.; Silathapanasakul, A.; Thakaeng, C.; Chanasakulniyom, M.; Songtawee, N.; Porntadavity, S.; Pothacharoen, P.; Pruksakorn, D.; Kongtawelert, P.; Yenchitsomanus, P.T.; et al. Extracellular Vesicle-Mediated IL-1 Signaling in Response to Doxorubicin Activates PD-L1 Expression in Osteosarcoma Models. Cells 2022, 11, 1042. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Mazurakova, A.; Samec, M.; Koklesova, L.; Zhai, K.; Al-Ishaq, R.; Kajo, K.; Biringer, K.; Vybohova, D.; Brockmueller, A.; et al. Flavonoids against non-physiologic inflammation attributed to cancer initiation, development, and progression-3PM pathways. EPMA J. 2021, 12, 559–587. [Google Scholar] [CrossRef]
- El Menyiy, N.; El Allam, A.; Aboulaghras, S.; Jaouadi, I.; Bakrim, S.; El Omari, N.; Shariati, M.A.; Miftakhutdinov, A.; Wilairatana, P.; Mubarak, M.S.; et al. Inflammatory auto-immune diseases of the intestine and their management by natural bioactive compounds. Biomed. Pharmacother. 2022, 151, 113158. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barrado, M.J.; Iglesias-Osma, M.C.; Perez-Garcia, E.; Carrero, S.; Blanco, E.J.; Carretero-Hernandez, M.; Carretero, J. Role of Flavonoids in The Interactions among Obesity, Inflammation, and Autophagy. Pharmaceuticals 2020, 13, 342. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef]
- Sekaran, S.; Roy, A.; Thangavelu, L. Re-appraising the role of flavonols, flavones and flavonones on osteoblasts and osteoclasts—A review on its molecular mode of action. Chem. Biol. Interact. 2022, 355, 109831. [Google Scholar] [CrossRef]
- Dwi Antika, L.; Kim, Y.-H.; Kang, M.-K.; Park, S.-H.; Lee, E.-J.; Choi, Y.-J.; Kang, Y.-H. Dietary compound gossypetin inhibits bone resorption through down-regulating lysosomal cathepsin K activity and autophagy-related protein induction in actin ring-bearing osteoclasts. J. Funct. Foods 2016, 24, 390–402. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Ge, R.; Zhang, J.; Liu, W.; Mou, K.; Lv, S.; Mu, X. Gossypetin Inhibits Solar-UV Induced Cutaneous Basal Cell Carcinoma through Direct Inhibiting PBK/TOPK Protein Kinase. Anticancer Agents Med. Chem. 2019, 19, 1029–1036. [Google Scholar] [CrossRef]
- Khan, A.; Manna, K.; Bose, C.; Sinha, M.; Das, D.K.; Kesh, S.B.; Chakrabarty, A.; Banerji, A.; Dey, S. Gossypetin, a naturally occurring hexahydroxy flavone, ameliorates gamma radiation-mediated DNA damage. Int. J. Radiat. Biol. 2013, 89, 965–975. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Tome, S.M.; Oliveira, E.F.T.; Viegas, M.F.; Araujo, A.N.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; et al. Evaluation of a flavonoids library for inhibition of pancreatic alpha-amylase towards a structure-activity relationship. J. Enzyme Inhib. Med. Chem. 2019, 34, 577–588. [Google Scholar] [CrossRef]
- Gomes, A.; Neuwirth, O.; Freitas, M.; Couto, D.; Ribeiro, D.; Figueiredo, A.G.; Silva, A.M.; Seixas, R.S.; Pinto, D.C.; Tome, A.C.; et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorg. Med. Chem. 2009, 17, 7218–7226. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehar, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Masuda, H.; Miller, C.; Koeffler, H.P.; Battifora, H.; Cline, M.J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc. Natl. Acad. Sci. USA 1987, 84, 7716–7719. [Google Scholar] [CrossRef]
- Diller, L.; Kassel, J.; Nelson, C.E.; Gryka, M.A.; Litwak, G.; Gebhardt, M.; Bressac, B.; Ozturk, M.; Baker, S.J.; Vogelstein, B.; et al. p53 functions as a cell cycle control protein in osteosarcomas. Mol. Cell. Biol. 1990, 10, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.W.; Aslo, A.; Tsay, C.; Slamon, D.; Ishizaki, K.; Toguchida, J.; Yamamuro, T.; Lampkin, B.; Koeffler, H.P. Frequency and structure of p53 rearrangements in human osteosarcoma. Cancer Res. 1990, 50, 7950–7954. [Google Scholar]
- Oshima, Y.; Sasaki, Y.; Negishi, H.; Idogawa, M.; Toyota, M.; Yamashita, T.; Wada, T.; Nagoya, S.; Kawaguchi, S.; Yamashita, T.; et al. Antitumor effect of adenovirus-mediated p53 family gene transfer on osteosarcoma cell lines. Cancer Biol. Ther. 2007, 6, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, R.; Marcatti, M.; Drago-Ferrante, R.; D’Anneo, A.; Giuliano, M.; Carlisi, D.; De Blasio, A.; Querques, F.; Pastore, L.; Tesoriere, G.; et al. Mutant p53 gain of function can be at the root of dedifferentiation of human osteosarcoma MG63 cells into 3AB-OS cancer stem cells. Bone 2014, 60, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.P.; Zhou, Y.; Gupta, A.; Leong, D.T.; Aung, K.Z.; Ling, L.; Pho, R.W.; Galindo, M.; Salto-Tellez, M.; Stein, G.S.; et al. Runx2, p53, and pRB status as diagnostic parameters for deregulation of osteoblast growth and differentiation in a new pre-chemotherapeutic osteosarcoma cell line (OS1). J. Cell. Physiol. 2009, 221, 778–788. [Google Scholar] [CrossRef]
- Ottaviano, L.; Schaefer, K.L.; Gajewski, M.; Huckenbeck, W.; Baldus, S.; Rogel, U.; Mackintosh, C.; de Alava, E.; Myklebost, O.; Kresse, S.H.; et al. Molecular characterization of commonly used cell lines for bone tumor research: A trans-European EuroBoNet effort. Genes Chromosomes Cancer 2010, 49, 40–51. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L.; et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Fukumoto, S.; Matsumoto, T. Relationship between actions of transforming growth factor (TGF)-beta and cell surface expression of its receptors in clonal osteoblastic cells. J. Cell. Physiol. 1995, 162, 315–321. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, J.M.P.; Almeida, J.F.D.; Martins, M.; Proenca, C.; Oliveira, H.; Fernandes, E.; Santos, C. 3′,4′-Dihydroxyflavonol Modulates the Cell Cycle in Cancer Cells: Implication as a Potential Combination Drug in Osteosarcoma. Pharmaceuticals 2021, 14, 640. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, J.M.P.; Pacheco, A.R.; Coutinho, L.; Oliveira, H.; Pinho, S.; Almeida, L.; Fernandes, E.; Santos, C. Combination of etoposide and fisetin results in anti-cancer efficiency against osteosarcoma cell models. Arch. Toxicol. 2018, 92, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Oliveira, J.M.P.; Proença, C.; Rufino, A.T.; Santos, I.; Fernandes, E. Osteosarcoma cell density upon 48h incubation with luteolin derivatives. Mendeley Data 2023. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Yin, F.; Guo, P.; Yang, X.; Liu, J.; Han, Y.; Ren, Z. Systemic Inflammatory Markers for Predicting Overall Survival in Patients with Osteosarcoma: A Systematic Review and Meta-Analysis. Mediators Inflamm. 2021, 2021, 3456629. [Google Scholar] [CrossRef]
- Han, Y.; Guo, W.; Ren, T.; Huang, Y.; Wang, S.; Liu, K.; Zheng, B.; Yang, K.; Zhang, H.; Liang, X. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019, 440–441, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Ribeiro, D.; Tomé, S.M.; Silva, A.M.; Fernandes, E. Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur. J. Med. Chem. 2014, 86, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Ribeiro, D.; Soares, T.; Tomé, S.M.; Silva, A.M.S.; Lima, J.; Fernandes, E.; Freitas, M. Chlorinated Flavonoids Modulate the Inflammatory Process in Human Blood. Inflammation 2017, 40, 1155–1165. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, X.; Xia, H. The flavonoid luteolin enhances doxorubicin-induced autophagy in human osteosarcoma U2OS cells. Int. J. Clin. Exp. Med. 2015, 8, 15190–15197. [Google Scholar]
- Wang, Y.; Kong, D.; Wang, X.; Dong, X.; Tao, Y.; Gong, H. Molecular mechanisms of luteolin induced growth inhibition and apoptosis of human osteosarcoma cells. Iran. J. Pharm. Res. 2015, 14, 531–538. [Google Scholar]
- Berndt, K.; Campanile, C.; Muff, R.; Strehler, E.; Born, W.; Fuchs, B. Evaluation of quercetin as a potential drug in osteosarcoma treatment. Anticancer Res. 2013, 33, 1297–1306. [Google Scholar] [PubMed]
- Catanzaro, D.; Ragazzi, E.; Vianello, C.; Caparrotta, L.; Montopoli, M. Effect of Quercetin on Cell Cycle and Cyclin Expression in Ovarian Carcinoma and Osteosarcoma Cell Lines. Nat. Prod. Commun. 2015, 10, 1365–1368. [Google Scholar] [CrossRef]
- Jing, D.; Wu, W.; Chen, X.; Xiao, H.; Zhang, Z.; Chen, F.; Zhang, Z.; Liu, J.; Shao, Z.; Pu, F. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 Via the JAK2-STAT3-PDL1. Pharmacol. Res. 2022, 182, 106287. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed. Pharmacother. 2019, 114, 108839. [Google Scholar] [CrossRef]
- Liang, W.; Li, X.; Li, C.; Liao, L.; Gao, B.; Gan, H.; Yang, Z.; Liao, L.; Chen, X. Quercetin-mediated apoptosis via activation of the mitochondrial-dependent pathway in MG-63 osteosarcoma cells. Mol. Med. Rep. 2011, 4, 1017–1023. [Google Scholar] [CrossRef]
- Xie, X.; Yin, J.; Jia, Q.; Wang, J.; Zou, C.; Brewer, K.J.; Colombo, C.; Wang, Y.; Huang, G.; Shen, J. Quercetin induces apoptosis in the methotrexate-resistant osteosarcoma cell line U2-OS/MTX300 via mitochondrial dysfunction and dephosphorylation of Akt. Oncol. Rep. 2011, 26, 687–693. [Google Scholar] [CrossRef]
- Wu, B.; Zeng, W.; Ouyang, W.; Xu, Q.; Chen, J.; Wang, B.; Zhang, X. Quercetin induced NUPR1-dependent autophagic cell death by disturbing reactive oxygen species homeostasis in osteosarcoma cells. J. Clin. Biochem. Nutr. 2020, 67, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Q.; Chen, J.; Chen, Z. Quercetin Enhances Cisplatin Sensitivity of Human Osteosarcoma Cells by Modulating microRNA-217-KRAS Axis. Mol. Cells 2015, 38, 638–642. [Google Scholar] [CrossRef]
- Xie, X.; Liu, K.; Liu, F.; Chen, H.; Wang, X.; Zu, X.; Ma, X.; Wang, T.; Wu, Q.; Zheng, Y.; et al. Gossypetin is a novel MKK3 and MKK6 inhibitor that suppresses esophageal cancer growth in vitro and in vivo. Cancer Lett. 2019, 442, 126–136. [Google Scholar] [CrossRef]
- Lee, M.S.; Tsai, C.W.; Wang, C.P.; Chen, J.H.; Lin, H.H. Anti-prostate cancer potential of gossypetin via inducing apoptotic and autophagic cell death. Mol. Carcinog. 2017, 56, 2578–2592. [Google Scholar] [CrossRef]
- Shao, H.; Ge, M.; Zhang, J.; Zhao, T.; Zhang, S. Osteoclasts differential-related prognostic biomarker for osteosarcoma based on single cell, bulk cell and gene expression datasets. BMC Cancer 2022, 22, 288. [Google Scholar] [CrossRef]
- Nørregaard, K.S.; Jürgensen, H.J.; Gårdsvoll, H.; Engelholm, L.H.; Behrendt, N.; Søe, K. Osteosarcoma and Metastasis Associated Bone Degradation—A Tale of Osteoclast and Malignant Cell Cooperativity. Int. J. Mol. Sci. 2021, 22, 6865. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Fernandes, A.; Fernandes, M.H. Reciprocal osteoblastic and osteoclastic modulation in co-cultured MG63 osteosarcoma cells and human osteoclast precursors. J. Cell. Biochem. 2011, 112, 3704–3713. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Teixeira, C.A.; Fernandes, M.H. Paracrine-mediated osteoclastogenesis by the osteosarcoma MG63 cell line: Is RANKL/RANK signalling really important? Clin. Exp. Metastasis 2011, 28, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-Gamma-Mediated Osteoimmunology. Front. Immunol. 2018, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Billiau, A.; Edy, V.G.; Heremans, H.; Van Damme, J.; Desmyter, J.; Georgiades, J.A.; De Somer, P. Human interferon: Mass production in a newly established cell line, MG-63. Antimicrob. Agents Chemother. 1977, 12, 11–15. [Google Scholar] [CrossRef]
- Martínez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of Flavonoids and Its Derivatives on Immune Cell Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, G.; Du, L. Avicularin Reduces the Expression of Mediators of Inflammation and Oxidative Stress in Bradykinin-Treated MG-63 Human Osteoblastic Osteosarcoma Cells. Med. Sci. Monit. 2020, 26, e921957. [Google Scholar] [CrossRef]
- Khan, A.; Manna, K.; Das, D.K.; Kesh, S.B.; Sinha, M.; Das, U.; Biswas, S.; Sengupta, A.; Sikder, K.; Datta, S.; et al. Gossypetin ameliorates ionizing radiation-induced oxidative stress in mice liver--a molecular approach. Free Radic. Res. 2015, 49, 1173–1186. [Google Scholar] [CrossRef]
- Han, Z.P.; Liu, D.B.; Wu, L.Q.; Li, Q.; Wang, Z.G.; Zang, X.F. IL-1β secreted by macrophage M2 promotes metastasis of osteosarcoma via NF-κB/miR-181α-5p/RASSF1A/Wnt pathway. Transl. Cancer Res. 2020, 9, 2721–2733. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, X.; Liu, Y.; Tang, S.; Miao, J.; Zhou, Q.; Chen, S. IL-1β-induced NF-κB activation down-regulates miR-506 expression to promotes osteosarcoma cell growth through JAG1. Biomed. Pharmacother. 2017, 95, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jin, X.; Cao, B.; Wang, W. Berberine affects osteosarcoma via downregulating the caspase-1/IL-1β signaling axis. Oncol. Rep. 2017, 37, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, K.; Nanjan, P.; Shaji, S.K.; Sunilkumar, D.; Subhalakshmi, K.; Rajakrishna, L.; Banerji, A. Discovery of lesser known flavones as inhibitors of NF-kappaB signaling in MDA-MB-231 breast cancer cells—A SAR study. Bioorg. Med. Chem. Lett. 2014, 24, 4735–4742. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Nair, A.S.; Ahn, K.S.; Pandey, M.K.; Yi, Z.; Liu, M.; Aggarwal, B.B. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappaB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood 2007, 109, 5112–5121. [Google Scholar] [CrossRef] [PubMed]







 | WST-8 assay (IC50 ± SEM) | SRB assay (IC50 ± SEM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | R3 | R6 | R8 | MG-63 | Saos-2 | HOS | 143B | MG-63 | Saos-2 | HOS | 143B |
| A1 | H | H | H | 83 ± 2 | 67 ± 4 | 59 ± 2 | 81 ± 3 | 67 ± 4 | 55 ± 6 | 61 ± 2 | 61 ± 4 |
| A2 | OH | H | H | 142 ± 8 | 70 ± 4 | 72 ± 5 | 117 ± 10 | 113 ± 10 | 61 ± 5 | 81 ± 4 | 88 ± 23 |
| A3 | OH | H | OH | 27 ± 2 | 38 ± 1 | 12 ± 1 | 35 ± 6 | 24 ± 2 | 30 ± 3 | 11 ± 1 | 33 ± 5 |
| B1 | Cl | H | H | 56 ± 6 | 62 ± 4 | 60 ± 2 | 49 ± 5 | 37 ± 2 | 37 ± 4 | 18 ± 2 | 21 ± 1 |
| B2 | Cl | H | Cl | 128 ± 10 | 109 ± 6 | 128 ± 17 | 80 ± 5 | 95 ± 2 | 87 ± 2 | 31 ± 5 | 40 ± 3 |
| B3 | H | Cl | H | 93 ± 2 | 98 ± 5 | 37 ± 3 | 49 ± 4 | 78 ± 9 | 68 ± 5 | 34 ± 1 | 27 ± 2 |
| B4 | H | Cl | Cl | 126 ± 2 | 116 ± 6 | 81 ± 8 | 79 ± 3 | 127 ± 4 | 99 ± 8 | 72 ± 2 | 64 ± 5 |
| C1 |  | H | H | 125 ± 14 | 84 ± 7 | 64 ± 7 | 94 ± 5 | 79 ± 2 | 56 ± 7 | 25 ± 4 | 37 ± 3 |
| C2 |  | H | H | 100 ± 4 | 74 ± 9 | 62 ± 1 | 58 ± 2 | 75 ± 9 | 68 ± 4 | 32 ± 1 | 37 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proença, C.; Rufino, A.T.; Santos, I.; Albuquerque, H.M.T.; Silva, A.M.S.; Fernandes, E.; Ferreira de Oliveira, J.M.P. Gossypetin Is a Novel Modulator of Inflammatory Cytokine Production and a Suppressor of Osteosarcoma Cell Growth. Antioxidants 2023, 12, 1744. https://doi.org/10.3390/antiox12091744
Proença C, Rufino AT, Santos I, Albuquerque HMT, Silva AMS, Fernandes E, Ferreira de Oliveira JMP. Gossypetin Is a Novel Modulator of Inflammatory Cytokine Production and a Suppressor of Osteosarcoma Cell Growth. Antioxidants. 2023; 12(9):1744. https://doi.org/10.3390/antiox12091744
Chicago/Turabian StyleProença, Carina, Ana Teresa Rufino, Isabela Santos, Hélio M. T. Albuquerque, Artur M. S. Silva, Eduarda Fernandes, and José Miguel P. Ferreira de Oliveira. 2023. "Gossypetin Is a Novel Modulator of Inflammatory Cytokine Production and a Suppressor of Osteosarcoma Cell Growth" Antioxidants 12, no. 9: 1744. https://doi.org/10.3390/antiox12091744
APA StyleProença, C., Rufino, A. T., Santos, I., Albuquerque, H. M. T., Silva, A. M. S., Fernandes, E., & Ferreira de Oliveira, J. M. P. (2023). Gossypetin Is a Novel Modulator of Inflammatory Cytokine Production and a Suppressor of Osteosarcoma Cell Growth. Antioxidants, 12(9), 1744. https://doi.org/10.3390/antiox12091744