Ozone Exposure Controls Oxidative Stress and the Inflammatory Process of Hepatocytes in Murine Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focus Question
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction and Management
2.5. Bias Analysis
3. Results
3.1. Selection of PRISMA-Guided Studies
3.2. Animal Model Characteristics
3.3. Methods Used to Cause Liver Injury
3.4. Ozone Exposure Characteristics
3.5. Outcomes
3.5.1. Ozone Exposure and Metabolism Redox
3.5.2. Ozone Exposure and Inflammation
3.5.3. Secondary Outcomes
3.5.4. Risk of Bias and Methodological Quality Assessments
4. Discussion
4.1. Characteristics of the Study and the Animal Model
4.2. Intervention Characteristics
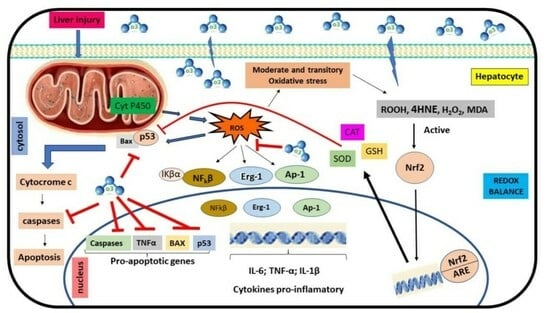
4.3. Ozone Exposure and Redox Metabolism and Inflammation Process
4.4. Ozone Exposure and Other Markers
4.5. Methodological Quality and Risk of Bias
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, K.; Chandra, S.; Gourisaria, M.K. Early-stage detection of liver disease through machine learning algorithms BT-advances indata and information sciences. In Advances in Data and Information Sciences; Tiwari, S., Trivedi, M.C., Kolhe, M.L., Mishra, K.K., Singh, B.K., Eds.; Springer: Singapore, 2022; Volume 318, pp. 155–166. [Google Scholar]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Uchida, D.; Takaki, A.; Oyama, A.; Adachi, T.; Wada, N.; Onishi, H.; Okada, H. Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 2020, 12, 1576. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Güvendi, G.F.; Eroğlu, H.A.; Makav, M.; Güvendi, B.; Adalı, Y. Selenium or ozone: Effects on liver injury caused by experimental iron overload. Life Sci. 2020, 262, 118558. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Viñals, M.; Isidoro, T.; Vilás, F. Potential role of oxygen–ozone therapy in treatment of covid-19 pneumonia. Am. J. Case Rep. 2020, 21, e925849. [Google Scholar] [CrossRef]
- Scassellati, C.; Ciani, M.; Galoforo, A.C.; Zanardini, R.; Bonvicini, C.; Geroldi, C. Molecular mechanisms in cognitive frailty: Potential therapeutic targets for oxygen-ozone treatment. Mech. Ageing Dev. 2020, 186, 111210. [Google Scholar] [CrossRef] [PubMed]
- Galiè, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The role of Nrf2 in the antioxidant cellular response to medical ozone exposure. Int. J. Mol. Sci. 2019, 20, 4009. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: A natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res. Rev. 2020, 63, 101138. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. Syrcle’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 1471–2288. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Akgul, H.; Selamoglu, Z. The effects on oxidative systems in liver tissues of systemic ozone application after critical size bone defect surgery in rat mandibles. Rom. Biotechnol. Lett. 2019, 2, 538–544. [Google Scholar] [CrossRef]
- Laszczyca, P.; Kawka-Serwecińska, E.; Witas, I.; Dolezych, B.; Falkus, B.; Mekail, A.; Ziółkowska, B.; Madej, P.; Migula, P. Lipid peroxidation and activity of antioxidative enzymes in the rat model of ozone therapy. Pol. J. Pharmacol. 1996, 28, 155–160. [Google Scholar]
- León, O.S.; Menéndez, S.; Merino, N.; Castillo, R.; Sam, S.; Pérez, L.; Bocci, V. Ozone oxidative preconditioning: A protection against cellular damage by free radicals. Mediators Inflamm. 1988, 7, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Leon, O.S.; Xaus, C.; Prats, N.; Jalil, E.C.; Planell, E.S.; Parellada, P.P.; Gelpí, E.; Roselló-Catafau, J. Protective effect of ozone treatment on the injury associated with hepatic ischemia-reperfusion: Antioxidant-prooxidant balance. Free Rad. Res. 1999, 31, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Xaus, C.; Bartrons, R.; Leon, O.S.; Gelpí, E.; Roselló-Catafau, J. Effect of ozone treatment on reactive oxygen species and adenosine production during hepatic ischemia-reperfusion. Free Rad. Res. 2000, 33, 595–605. [Google Scholar] [CrossRef]
- Jalil, E.C.; Mohammed-Al-Dalain, S.; Fernández, O.L.; Menendez, S.; Pérez-Davison, G.; Merino, N.; Sam, S.; Ajamieh, H.H. Oxidative preconditioning affords protection against carbon tetrachloride-induced glycogen depletion and oxidative stress in rats. J. Appl. Toxicol. 2001, 21, 297–301. [Google Scholar] [CrossRef]
- Ajamieh, H.; Merino, N.; Jalil, E.C.; Menéndez, S.; Martinez-Sanchez, G.; Re, L.; Giuliani, A.; Leon, O.S. Similar protective effect of ischaemic and ozone oxidative preconditionings in liver ischaemia/reperfusion injury. Pharmacol. Res. 2002, 45, 333–339. [Google Scholar] [CrossRef]
- Ajamieh, H.H.; Menéndez, S.; Martínez-Sánchez, G.; Jalil, E.C.; Re, L.; Giuliani, A.; Fernández, O.S.L. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia–reperfusion. Liver Int. 2004, 24, 55–62. [Google Scholar] [CrossRef]
- Ajamieh, H.H.; Berlanga, J.; Merino, N.; Sanchez, G.M.; Carmona, A.M.; Cepero, S.M.; Giuliani, A.; Re, L.; Leon, O.S. Role of protein synthesis in the protection conferred by ozone-oxidative-preconditioning in hepatic ischaemia/reperfusion. Transpl. Int. 2005, 18, 604–612. [Google Scholar] [CrossRef]
- Zamora, Z.B.; Borrego, A.; López, O.Y.; Delgado, R.; González, R.; Menéndez, S.; Hernández, F.; Schulz, S. Effects of ozone oxidative preconditioning on TNF-α release and antioxidant-prooxidant intracellular balance in mice during endotoxic shock. Mediators Inflamm. 2005, 2005, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Madej, P.; Plewka, A.; Madej, J.A.; Nowak, M.; Plewka, D.; Franik, G.; Golka, D. Ozone therapy in an induced septic shock. I. Effect of ozonotherapy on rat organs in the evaluation of free radical reactions and selected enzymatic systems. Inflammation 2007, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Guanche, D.; Hernandez, F.; Zamora, Z.; Alonso, Y. Effect of ozone pre-conditioning on redox activity in a rat model of septic shock. Toxicol. Mech. Methods 2010, 20, 466–471. [Google Scholar] [CrossRef]
- Rodríguez, Z.Z.; Guanch, D.; Álvarez, R.G.; Martinez, Y.; Alonso, Y.; Schul, S. Effects of ozone oxidative preconditioning on different hepatic biomarkers of oxidative stress in endotoxic shock in mice. Toxicol. Mech. Methods 2011, 21, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Uysa, B.; Cakir, E.; Yaman, H.; Macit, E.; Yildirim, A.O.; Eyi, Y.E.; Kaldirim, U.; Oztas, E.; Akgul, E.O.; et al. The protective effects of ozone therapy in a rat model of acetaminophen-induced liver injury. Environ. Toxicol. Pharmacol. 2012, 34, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, F.A.; Bakkal, B.H.; Guven, B.; Tasdoven, I.; Bektas, S.; Can, M.; Comert, M. Effects of ozone oxidative preconditioning on radiation-induced organ damage in rats. J. Radiat. Res. 2013, 54, 36–44. [Google Scholar] [CrossRef]
- Safwat, M.H.; El-Sawalhi, M.M.; Mausouf, M.N.; Shaheen, A.A. Ozone ameliorates age-related oxidative stress changes in rat liver and kidney: Effects of pre- and post-ageing administration. Biochemistry 2014, 79, 450–458. [Google Scholar] [CrossRef]
- Aslaner, A.; Çakır, T.; Çelik, B.; Doğan, U.; Akyüz, C.; Baştürk, A.; Polat, C.; Gündüz, U.; Mayir, B.; Şehirli, A.Ö. The protective effect of intraperitoneal medical ozone preconditioning and treatment on hepatotoxicity induced by methotrexate. Int. J. Clin. Exp. Med. 2015, 8, 13303. [Google Scholar]
- Adali, Y.; Eroǧlu, H.A.; Makav, M.; Guvendi, G.F. Efficacy of ozone and selenium therapy for alcoholic liver injury: An experimental model. In Vivo 2019, 33, 763–769. [Google Scholar] [CrossRef]
- Ozger, H.S.; Dizbay, M.; Corbacioglu, S.K.; Aysert, P.; Demirbas, Z.; Tunccan, O.G.; Hizel, K.; Bozdayi, G.; Caglar, K. The prognostic role of neopterin in covid-19 patients. J. Med. Virol. 2021, 93, 1520–1525. [Google Scholar] [CrossRef]
- Dhillon, A.K.; Rupp, C.; Bergquist, A.; Voitl, R.; Folseraas, T.; Trøseid, M.; Midttun, Ø.; Ueland, P.M.; Karlsen, T.H.; Vesterhus, M.; et al. Associations of neopterin and kynurenine–tryptophan ratio with survival in primary sclerosing cholangitis. Scand. J. Gastroenterol. 2021, 56, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, Z.; Wei, Y. Advances in understanding mechanisms underlying mitochondrial structure and function damage by ozone. Sci. Total Environ. 2023, 861, 160589. [Google Scholar] [CrossRef]
- Yan, Z.; Jin, Y.; An, Z.; Liu, Y.; Samet, J.M.; Wu, W. Inflammatory cell signaling following exposures to particulate matter and ozone. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Valdenassi, L.; Simonetti, V.; Bertossi, D.; Ricevuti, G.; Franzini, M.; Pandolfi, S. Insights on the mechanisms of action of ozone in the medical therapy against COVID-19. Int. Immunopharmacol. 2021, 96, 107777. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Costanzo, M.; Cisterna, B.; Nodari, A.; Galiè, M.; Cattaneo, A.; Covi, V.; Tabaracci, G.; Bonvicini, C.; Malatesta, M. Effects of mild ozonisation on gene expression and nuclear domains organization in vitro. Toxicol. Vitr. 2017, 44, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Vinals, M.; Pablos, A.; Vilás, F.; Papadakos, P.J.; Wijeysundera, D.N.; Bergese, S.D.; Vives, M. Ozone therapy for patients with covid-19 pneumonia: Preliminary report of a prospective case-control study. Int. Immunopharmacol. 2021, 90, 107261. [Google Scholar] [CrossRef]
- Schwartz, A.; Güémez, F.A.; Nazarov, S.E.I.; Viebahn-Haensler, R.; Rieck, A.; Stefan, T. Madrid declaration on ozone therapy. In International Scientific Committee of Ozone Therapy, 3rd ed.; Madrid, Spain. 2020. Available online: www.isco3.org (accessed on 13 May 2021).
- Zucker, I.; Beery, A.K. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef]
- Beal, E.W.; Dumond, C.; Kim, J.L.; Akateh, C.; Eren, E.; Maynard, K.; Sen, C.K.; Zweier, J.L.; Washburn, K.; Whitson, B.A.; et al. A small animal model of ex vivo normothermic liver perfusion. J. Vis. Exp. 2018, 136, e57541. [Google Scholar] [CrossRef]
- Nogueira, B.C.F.; Campos, A.K.; Alves, R.S.; Sarandy, M.M.; Novaes, R.D.; Esposito, D.; Gonçalves, R.V. What is the impact of depletion of immunoregulatory genes on wound healing? a systematic review of preclinical evidence. Oxid. Med. Cell. Longev. 2020, 2020, 8862953. [Google Scholar] [CrossRef]
- Basauri, A.; González-Fernández, C.; Fallanza, M.; Bringas, E.; Fernandez-Lopez, R.; Giner, L.; Moncalián, G.; de la Cruz, F.; Ortiz, I. Biochemical interactions between LPS and LPS-binding molecules. Crit. Rev. Biotechnol. 2020, 40, 292–305. [Google Scholar] [CrossRef]
- Davydova, E.V.; Osikov, M.V.; Kaygorodtseva, N.V. Effect of local ozone therapy on inflammatory markers in experimental ulcerative colitis. Bull. Sib. Med. 2022, 21, 47–53. [Google Scholar] [CrossRef]
- Toman, H.; Sahin, H.; Erbas, M.; Turkon, H.; Simsek, T.; Kiraz, H.A.; Özkan, M.T.A. Effects of prophylactic ozone therapy on general anesthesia and surgical stress response: Neutrophil/lymphocyte ratio and ischemia-modified albumin. Int. Surg. 2019, 104, 467–473. [Google Scholar] [CrossRef]
- Yousefi, B.; Banihashemian, S.Z.; Feyzabadi, Z.K.; Hasanpour, S.; Kokhaei, P.; Abdolshahi, A.; Emadi, A.; Eslami, M. Potential therapeutic effect of oxygen-ozone in controlling of covid-19 disease. Med. Gas Res. 2022, 12, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Cisterna, B.; Vella, A.; Cestari, T.; Covi, V.; Tabaracci, G.; Malatesta, M. Low ozone concentrations stimulate cytoskeletal organization.; mitochondrial activity and nuclear transcription. Eur. J. Histochem. 2015, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Viebahn-Haensler, R.; León Fernández, O.S. Ozone in medicine. The low-dose ozone concept and its basic biochemical mechanisms of action in chronic inflammatory diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.C.; Grillo, R.; de Barros, T.E.P.; Martins, C.B.; de Carvalho Luposeli, F. Efficacy of biostimulatory ozone therapy: Case report and literature review. J. Cosmet. Dermatol. 2022, 21, 130–133. [Google Scholar] [CrossRef]
- Huang, Y.J.; Nan, G.X. Oxidative stress-induced angiogenesis. J. Clin. Neurosci. 2019, 63, 13–16. [Google Scholar] [CrossRef]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between oxidative stress and inflammatory liver injury in the pathogenesis of alcoholic liver Disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef]
- Clavo, B.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Martínez-Sánchez, G.; Llontop, P.; Aguiar-Bujanda, D.; Fernández-Pérez, L.; Santana-Rodríguez, N. Modulation of oxidative stress by ozone therapy in the prevention and treatment of chemotherapy-induced toxicity: Review and prospects. Antioxidants 2019, 8, 588. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, M.; Zhou, B.; Cai, P.; Larsson, T.E.; Zhao, J.; Bowden, T.M. Weak acidic stable carbazate modified cellulose membranes target for scavenging carbonylated proteins in hemodialysis. Carbohydr. Polym. 2020, 231, 115727. [Google Scholar] [CrossRef]
- Ozturk, O.; Eroglu, H.A.; Ustebay, S.; Kuzucu, M.; Adali, Y. An experimental study on the preventive effects of n-acetyl cysteine and ozone treatment against contrast-induced nephropathy. Acta Cir. Bras. 2018, 33, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Sarandy, M.M.; Novaes, R.D.; Xavier, A.A.; Vital, C.E.; Leite, J.P.; Melo, F.C.; Gonçalves, R.V. Hydroethanolic extract of strychnos pseudoquina accelerates skin wound healing by modulating the oxidative status and microstructural reorganization of scar tissue in experimental type i diabetes. BioMed Res. Int. 2017, 2017, 9538351. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Skóra, B.; Balawejder, M. Ozone treatment induces changes in antioxidative defense system in blueberry fruit during storage. Food Bioprocess Technol. 2020, 13, 1240–1245. [Google Scholar] [CrossRef]
- Prieto-Bermejo, R.; Hernández-Hernández, A. The importance of nadph oxidases and redox signaling in angiogenesis. Antioxidants 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Sirokmány, G.; Geiszt, M. The relationship of nadph oxidases and heme peroxidases: Fallin’ in and out. Front. Immunol. 2019, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Cenci, A.; Macchia, I.; La Sorsa, V.; Sbarigia, C.; Di Donna, V.; Pietraforte, D. Mechanisms of action of ozone therapy in emerging viral diseases: Immunomodulatory effects and therapeutic advantages with reference to SARS-CoV-2. Front. Microbiol. 2022, 13, 871645. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Feng, H.; Jia, X.M.; Tang, H.; Liao, Y.Y.; Li, B.R. Ozone therapy ameliorates inflammation and endometrial injury in rats with pelvic inflammatory disease. Biomed. Pharmacother. 2018, 107, 1418–1425. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef]
- Oliva-Vilarnau, N.; Hankeova, S.; Vorrink, S.U.; Mkrtchian, S.; Andersson, E.R.; Lauschke, V.M. Calcium signaling in liver injury and regeneration. Front. Med. 2018, 5, 192. [Google Scholar] [CrossRef]
- Hossain, K.R.; Li, X.; Zhang, T.; Paula, S.; Cornelius, F.; Clarke, R.J. Polarity of the ATP binding site of the Na+, K+-ATPase, gastric H+, K+-ATPase and sarcoplasmic reticulum Ca2+-ATPase. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183138. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, S.; Albini, S.; Best, H.; Richard, I. Calpains for dummies: What you need to know about the calpain family. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140616. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, Y. Hepatoprotective effects of sophoricoside against fructose-induced liver injury via regulating lipid metabolism, oxidation, and inflammation in mice. J. Food Sci. 2018, 83, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, L.; Xu, D.; Deng, W.; Yang, W.; Tang, F.; Da, M. Knockout of calpain-1 protects against high-fat diet-induced liver dysfunction in mouse through inhibiting oxidative stress and inflammation. Food Sci. Nutr. 2021, 9, 367–374. [Google Scholar] [CrossRef]
- Sharma, A.; Jaiswal, P.; Kerakhan, Y.; Saravanan, L.; Murtaza, Z.; Zergham, A.; Honganur, N.S.; Akbar, A.; Deol, A.; Francis, B.; et al. Liver disease and outcomes among covid-19 hospitalized patients—A systematic review and meta-analysis. Ann. Hepatol. 2021, 21, 100273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.B.; Gao, J.; Chai, Y.H.; Li, W.; Luo, Y.F.; Chen, Y.Z. Astragaloside alleviates alcoholic fatty liver disease by suppressing oxidative stress. Kaohsiung J. Med. Sci. 2021, 37, 718–729. [Google Scholar] [CrossRef]
- Kagawa, T.; Shirai, Y.; Oda, S.; Yokoi, T. Identification of specific microrna biomarkers in early stages of hepatocellular injury, cholestasis, and steatosis in rats. Toxicol. Sci. 2018, 166, 228–239. [Google Scholar] [CrossRef]
- Sharma, S.; Raghuvanshi, S.; Jaswal, A.; Shrivastava, S.; Shukla, S. Lead acetate-induced hepatoxicity in wistar rats: Possible protective role of combination therapy. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 23–34. [Google Scholar] [CrossRef]
- Malinska, D.; Testoni, G.; Duran, J.; Brudnicka, A.; Guinovart, J.J.; Duszynski, J. Hallmarks of oxidative stress in the livers of aged mice with mild glycogen branching enzyme deficiency. Arch. Biochem. Biophys. 2020, 695, 108626. [Google Scholar] [CrossRef]




| Oxidative Markers | Pro−Oxidant Enzymes | Antioxidant Markers | Infl. Cells | Infl. Mark | Morphologic Parameters | Other Analyzes | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDA | H2O2 | PC | 4−HDA | CD (Conjugado dienos) | NOX(NADPH OXIDASE) | XOD | MPO | SOD | Cu, Zn−SOD | Mn−SOD | CAT | GSH | GST | GSSG | GPX | Kupfer Cell | Neutrofilos | TNF−α | IL−1β | Necrosis | Degeneration | Congestion | periportal Inflammation | Ca−ATPase | Ca2+ | Calpain | Phospholipase A | Lipofuscin | Lactate | ATP + ADP | Ácido Urico | Glicogênio | Neopttirna | AST | ALT | |
| Laszczyca, et al., 1996 [14] | = | = | = | |||||||||||||||||||||||||||||||||
| León, et al., 1998 [15] | − | + | = | + | + | − | − | |||||||||||||||||||||||||||||
| Peralta, et al., 1999 [16] | − | + | + | − | − | − | − | − | ||||||||||||||||||||||||||||
| Peralta, et al., 2000 [17] | − | − | + | − | − | |||||||||||||||||||||||||||||||
| Jalil et al., 2001 [18] | − | + | = | − | − | + | ||||||||||||||||||||||||||||||
| Ajamieh et al., 2002 [19] | − | − | − | − | − | − | − | |||||||||||||||||||||||||||||
| Ajamieh et al., 2004 [20] | − | − | + | − | + | − | − | − | ||||||||||||||||||||||||||||
| Ajamieh et al., 2005 [21] | − | + | − | + | − | + | − | − | − | − | ||||||||||||||||||||||||||
| Zamora et al., 2005 [22] | − | − | + | + | − | |||||||||||||||||||||||||||||||
| Madej et al., 2007 [23] | − | − | ||||||||||||||||||||||||||||||||||
| Guanche et al., 2010 [24] | − | − | + | + | + | |||||||||||||||||||||||||||||||
| Rodríguez et al., 2011 [25] | − | + | + | + | ||||||||||||||||||||||||||||||||
| Gul et al., 2012 [26] | − | − | + | + | − | − | − | − | ||||||||||||||||||||||||||||
| Gultekin et al., 2012 [27] | − | + | − | − | − | − | − | − | − | |||||||||||||||||||||||||||
| Safwat et al., 2014 [28] | − | − | + | − | − | |||||||||||||||||||||||||||||||
| Aslaner et al., 2015 [29] | − | − | + | − | − | − | − | − | − | − | − | |||||||||||||||||||||||||
| Erdemli et al., 2019 [13] | − | − | + | + | ||||||||||||||||||||||||||||||||
| Adali et al., 2019 [30] | − | − | − | − | − | |||||||||||||||||||||||||||||||
| Guvendi et al., 2020 [5] | + | |||||||||||||||||||||||||||||||||||
| + | Increased |
| − | Reduced |
| = | Not effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelinsari, S.M.; Sarandy, M.M.; Vilela, E.F.; Novaes, R.D.; Schlamb, J.; Gonçalves, R.V. Ozone Exposure Controls Oxidative Stress and the Inflammatory Process of Hepatocytes in Murine Models. Antioxidants 2024, 13, 212. https://doi.org/10.3390/antiox13020212
Pelinsari SM, Sarandy MM, Vilela EF, Novaes RD, Schlamb J, Gonçalves RV. Ozone Exposure Controls Oxidative Stress and the Inflammatory Process of Hepatocytes in Murine Models. Antioxidants. 2024; 13(2):212. https://doi.org/10.3390/antiox13020212
Chicago/Turabian StylePelinsari, Silvania Mol, Mariáurea Matias Sarandy, Emerson Ferreira Vilela, Rômulo Dias Novaes, Jade Schlamb, and Reggiani Vilela Gonçalves. 2024. "Ozone Exposure Controls Oxidative Stress and the Inflammatory Process of Hepatocytes in Murine Models" Antioxidants 13, no. 2: 212. https://doi.org/10.3390/antiox13020212
APA StylePelinsari, S. M., Sarandy, M. M., Vilela, E. F., Novaes, R. D., Schlamb, J., & Gonçalves, R. V. (2024). Ozone Exposure Controls Oxidative Stress and the Inflammatory Process of Hepatocytes in Murine Models. Antioxidants, 13(2), 212. https://doi.org/10.3390/antiox13020212