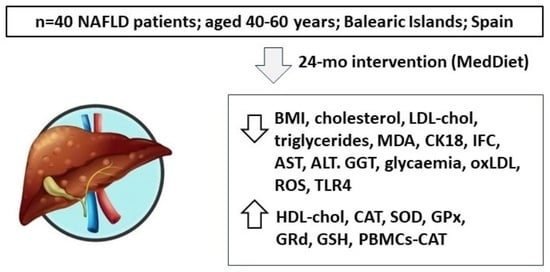
Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Anthropometrics, Clinical Assessment, and Physical Activity
2.4. NAFLD Diagnosis
2.5. Blood Collection and Analysis
2.6. Antioxidant Determinations and Antioxidant Score
2.7. Malondialdehyde Assay
2.8. Immunoassay Kits
2.9. ROS Production in PBMCs
2.10. RNA Extraction and Real-Time PCR
2.11. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J. Hepatol. 2013, 58, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Boqué, N.; del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 1052. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Day, C.P.; Dufour, J.F.; Canbay, A.; Nobili, V.; Ratziu, V.; Tilg, H.; Roden, M.; Gastaldelli, A.; Yki-Jarvinen, H.; et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lee, G.; Heo, S.Y.; Roh, Y.S. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple Hits, Including Oxidative Stress, as Pathogenesis and Treatment Target in Non-Alcoholic Steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Ekstedt, M.; Wong, G.L.H.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Igwe, M.C.; Obeagu, G.U. Oxidative stress’s impact on red blood cells: Unveiling implications for health and disease. Medicine 2024, 103, E37360. [Google Scholar] [CrossRef] [PubMed]
- Massaccesi, L.; Galliera, E.; Corsi Romanelli, M.M. Erythrocytes as markers of oxidative stress related pathologies. Mech. Ageing Dev. 2020, 191, 111333. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials.gov. US National Library of Medicine. Prevention and Reversion of NAFLD in Obese Patients with Metabolic Syndrome by Mediterranean Diet and Physical Activity (FLIPAN). Available online: https://clinicaltrials.gov/ct2/show/NCT04442620 (accessed on 18 May 2022).
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Bouzas, C.; Bibiloni, M.D.M.; Julibert, A.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Adherence to the Mediterranean Lifestyle and Desired Body Weight Loss in a Mediterranean Adult Population with Overweight: A PREDIMED-Plus Study. Nutrients 2020, 12, 2114. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Sirlin, C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 337–357. [Google Scholar] [CrossRef]
- Tang, A.; Tan, J.; Sun, M.; Hamilton, G.; Bydder, M.; Wolfson, T.; Gamst, A.C.; Middleton, M.; Brunt, E.M.; Loomba, R.; et al. Nonalcoholic Fatty Liver Disease: MR Imaging of Liver Proton Density Fat Fraction to Assess Hepatic Steatosis. Radiology 2013, 267, 422. [Google Scholar] [CrossRef]
- Bøyum, A. Separation of White Blood Cells. Nature 1964, 204, 793–794. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzym. Anal. 1984, 105, 121–126. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef] [PubMed]
- Flohe, L.; Gunzler, W. Assay of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [PubMed]
- Bergmayer, H.U. Glutathione reductase. Methods Enzym. Anal. Starch-Stärke 1963, 15, 272. [Google Scholar]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Int. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
- Salvoza, N.; Giraudi, P.J.; Tiribelli, C.; Rosso, N. Natural compounds for counteracting nonalcoholic fatty liver disease (NAFLD): Advantages and limitations of the suggested candidates. Int. J. Mol. Sci. 2022, 23, 2764. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Choghakhori, R.; Ashtary-Larky, D.; Abbasnezhad, A. Effects of the Mediterranean diet on cardiovascular risk factors in non-alcoholic fatty liver disease patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2020, 37, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Perna, S.; Allehdan, S.; Rafique, A.; Saad, S.; AlGhareeb, F.; Rondanelli, M.; Tayyem, R.F.; Marino, M.; Martini, D.; et al. Does the Mediterranean Diet Have Any Effect on Lipid Profile, Central Obesity and Liver Enzymes in Non-Alcoholic Fatty Liver Disease (NAFLD) Subjects? A Systematic Review and Meta-Analysis of Randomized Control Trials. Nutrients 2023, 15, 2250. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Bouzas, C.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Llompart, I.; Gámez, J.M.; Tejada, S.; Martínez, J.A.; et al. A Greater Improvement of Intrahepatic Fat Contents after 6 Months of Lifestyle Intervention Is Related to a Better Oxidative Stress and Inflammatory Status in Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 1266. [Google Scholar] [CrossRef]
- Zhong, F.; Guan, L.; Lin, H.; Zhao, M.; Qin, Y.; Li, Q.; Yuan, Z.; Yang, G.; Gao, L.; Zhao, J. Red Blood Cell Count: An Unrecognized Risk Factor for Nonalcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Abbate, M.; Montemayor, S.; Mascaró, C.M.; Casares, M.; Tejada, S.; Abete, I.; Zulet, M.A.; Tur, J.A.; et al. Oxidative stress and pro-inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants 2020, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Anandan, A.; Vrielink, A. Structure and function of lipid A–modifying enzymes. Ann. N. Y. Acad. Sci. 2020, 1459, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Baratta, F.; Pastori, D.; Cocomello, N.; Colantoni, A.; Angelico, F.; Del Ben, M. New Insights into the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Gut-Derived Lipopolysaccharides and Oxidative Stress. Nutrients 2020, 12, 2762. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Rafraf, M.; Farzin, L.; Asghari-Jafarabadi, M.; Ghavami, S.M.; Somi, M.H. Effects of Pharmacologic Dose of Resveratrol Supplementation on Oxidative/Antioxidative Status Biomarkers in Nonalcoholic Fatty Liver Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Pharm. Bull. 2018, 8, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Polito, A.; Fumagalli, A.; Intorre, F.; Venneria, E.; Durazzo, A.; Zaccaria, M.; Ciarapica, D.; Foddai, M.S.; Mauro, B.; et al. Mediterranean Diet Effect: An Italian picture. Nutr. J. 2011, 10, 125. [Google Scholar] [CrossRef]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.-C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795. [Google Scholar] [CrossRef]
- Ho, C.M.; Ho, S.L.; Jeng, Y.M.; Lai, Y.S.; Chen, Y.H.; Lu, S.C.; Chen, H.L.; Chang, P.Y.; Hu, R.H.; Lee, P.H. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J. Inflamm. 2019, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Castañer, O.; Goday, A.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; et al. The Mediterranean Diet decreases LDL atherogenicity in high cardiovascular risk individuals: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1601015. [Google Scholar] [CrossRef] [PubMed]
- Barona, J.; Jones, J.J.; Kopec, R.E.; Comperatore, M.; Andersen, C.; Schwartz, S.J.; Lerman, R.H.; Fernandez, M.L. A Mediterranean-style low-glycemic-load diet increases plasma carotenoids and decreases LDL oxidation in women with metabolic syndrome. J. Nutr. Biochem. 2012, 23, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Min, B.-H.; Ganesan, R.; Gebru, Y.A.; Sharma, S.P.; Park, E.; Won, S.-M.; Jeong, J.-J.; Lee, S.-B.; Cha, M.-G.; et al. Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role. Biomedicines 2022, 10, 550. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed. Pharmacother. 2020, 131, 110785. [Google Scholar] [CrossRef]



| Antioxidants | Quintiles | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| CAT (k/109 erythrocytes) | Cut-off points | <8.78 | 8.78–10.25 | 10.25–10.96 | 10.97–12.19 | >12.19 |
| SOD (pkat/109 erythrocytes) | <24.96 | 24.96–28.26 | 28.26–32.65 | 32.65–37.32 | >37.32 | |
| GPx (nkat/109 erythrocytes) | <43.87 | 43.86–49.40 | 49.40–52.66 | 52.66–57.08 | >57.08 | |
| GRd (nkat/109 erythrocytes) | <96.73 | 96.73–106.52 | 106.52–119.29 | 119.29–130.09 | >130.09 | |
| GSH (mmol/109 erythrocytes) | <4.69 | 4.69–6.12 | 6.12–7.07 | 7.07–9.13 | >9.13 |
| Gene | Primer | Conditions | |
|---|---|---|---|
| 18S | Fw: 5′-ATgTgAAgTCACTgTgCCAg Rv: 5′-gTgTAATCCgTCTCCACAgA | 95 °C 60 °C 72 °C | 10 s 10 s 15 s |
| CAT | Fw: 5′-TTTggCTACTTTgAggTCAC Rv: 5′-TCCCCATTTgCATTAACCAg | 95 °C 60 °C 72 °C | 10 s 10 s 15 s |
| MnSOD | Fw: 5′-CgTgCTCCCACACATCAATC Rv: 5′-TgAACgTCACCgAggAgAAg | 95 °C 60 °C 72 °C | 10 s 10 s 12 s |
| TLR4 | Fw: 5′-ggTCACCTTTTCTTgATTCCA Rv: 5′-TCAgAggTCCATCAAACATCAC | 95 °C 60 °C 72 °C | 10 s 10 s 15 s |
| GPx | Fw: 5′-TTCCCgggCAACCAgTTTg Rv: 5’-TTCACCTCTCACTTCTCgAA | 95 °C 63 °C 72 °C | 10 s 10 s 15 s |
| GRd | Fw: 5′-TCACgCAgTTACCAAAAggAAA Rv: 5′-CACACCCAAgTCCCCTgCATAT | 95 °C 63 °C 72 °C | 10 s 10 s 15 s |
| Low Adherence to MedDiet | High Adherence to MedDiet | p-Value | |||
|---|---|---|---|---|---|
| Baseline (n = 20) | 2-Year Change (n = 20) | Baseline (n = 20) | 2-Year Change (n = 20) | ||
| Anthropometry | |||||
| Weight (kg) | 88.8 ± 9.94 | 87.6 ± 10.2 * | 87.2 ± 9.1 | 81.7 ± 8.9 *# | <0.001 |
| BMI (kg/m2) | 32.6 ± 2.1 | 32.1 ± 2.2 * | 32.2 ± 2.3 | 30.2 ± 1.9 *# | <0.001 |
| Systolic BP (mmHg) | 135.3 ± 17.3 | 136.1 ± 14.6 | 136.6 ± 17.7 | 134.8 ± 21.3 | 0.536 |
| Diastolic BP (mmHg) | 82.0 ± 8.0 | 85.6 ± 10.8 * | 81.7 ± 9.5 | 83.8 ± 11.1 | 0.345 |
| Clinical parameters | |||||
| Glucose (mg/dL) | 109.8 ± 19.6 | 102.2 ± 14.7 | 109.4 ± 21.3 | 97.1 ± 15.1 * | 0.275 |
| HbA1c (%) | 6.0 ± 0.8 | 5.9 ± 0.7 | 5.96 ± 1.0 | 5.8 ± 0.7 | 0.642 |
| Cholesterol total (mg/dL) | 198.3 ± 35.0 | 186.9 ± 30.6 * | 208.4 ± 26.5 | 186.8 ± 22.0 * | 0.094 |
| HDL-c (mg/dL) | 41.9 ± 8.6 | 45.7 ± 13.1 * | 39.9 ± 7.4 | 44.4 ± 9.3 * | 0.806 |
| LDL-c (mg/dL) | 129.7 ± 30.5 | 119.1 ± 28.0 * | 134.1 ± 34.9 | 118.6 ± 29.1 * | 0.433 |
| Triglycerides (mg/dL) | 172.8 ± 43.3 | 153.6 ± 40.6 * | 179.8 ± 27.4 | 145.2 ± 32.5 * | 0.197 |
| CRP (mg/dL) | 0.6 ± 0.5 | 0.4 ± 0.4 | 0.5 ± 0.7 | 0.4 ± 0.5 | 0.617 |
| AST (U/L) | 26.5 ± 6.9 | 24.6 ± 6.9 | 27.4 ± 7.3 | 21.1 ± 6.0 * | 0.014 |
| ALT (U/L) | 36.5 ± 18.2 | 33.2 ± 18.1 | 37.0 ± 12.4 | 28.0 ± 7.7 * | 0.094 |
| GGT (U/L) | 7.8 ± 7.8 | 32.6 ± 9.2 | 37.8 ± 11.6 | 30.7 ± 10.7 * | 0.075 |
| IFC (%) | 16.1 ± 4.6 | 14.3 ± 4.5 | 16.6 ± 4.7 | 12.1 ± 5.3 * | 0.012 |
| Haematological parameters | |||||
| Haematocrit (%) | 42.9 ± 4.4 | 42.7 ± 4.1 | 44.5 ± 4.2 # | 44.2 ± 3.6 | 0.299 |
| Erythrocytes (106/μL) | 4.9 ± 0.4 | 4.8 ± 0.4 | 4.9 ± 0.4 | 4.8 ± 0.36 * | 0.332 |
| Leukocytes (103/μL) | 7.6 ± 1.6 | 7.2 ± 1.1 | 7.4 ± 2.0 | 7.4 ± 2.3 | 0.125 |
| Platelets (103/μL) | 245.1 ± 40.5 | 237.8 ± 49.4 | 236.7 ± 38.4 | 228.3 ± 37.7 | 0.705 |
| Neutrophils (103/μL) | 4.4 ± 1.0 | 4.02 ± 0.9 | 3.9 ± 1.3 | 3.9 ± 1.6 | 0.077 |
| Lymphocytes (103/μL) | 2.3 ± 0.6 | 2.3 ± 0.5 | 2.7 ± 0.7 | 2.7 ± 0.6 | 0.452 |
| Monocytes (103/μL) | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.198 |
| Eosinophils (103/μL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.916 |
| Basophils (103/μL) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.334 |
| Energy Expendidure | |||||
| Measured accelerometer (MET/day) | 1850 ± 243 | 1802 ± 237 | 1862 ± 342 | 1795 ± 230 | 0.915 |
| Low Adherence to MedDiet | High Adherence to MedDiet | p-Value | |||
|---|---|---|---|---|---|
| Baseline (n = 20) | 2-Year Change (n = 20) | Baseline (n = 20) | 2-Year Change (n = 20) | ||
| Antioxidants | |||||
| CAT (k/109 erythrocytes) | 10.0 ± 1.9 | 10.0 ± 1.6 | 9.8 ± 1.9 | 12.6 ± 1.8 *# | <0.001 |
| SOD (pkat/109 erythrocytes) | 26.8 ± 7.5 | 28.4 ± 3.0 | 27.8 ± 8.4 | 38.0 ± 2.8 *# | 0.002 |
| GPx (nkat/109 erythrocytes) | 46.2 ± 6.0 | 50.4 ± 8.3 * | 48.2 ± 7.6 | 57.8 ± 6.2 *# | 0.018 |
| GRd (nkat/109 erythrocytes) | 105.0 ± 15.3 | 109.4 ± 16.3 | 104.8 ± 21.2 | 134.0 ± 13.5 *# | 0.001 |
| GSH (mmol/109 erythrocytes) | 5.8 ± 2.7 | 6.8 ± 1.9 | 6.0 ± 2.3 | 8.5 ± 3.2 *# | 0.240 |
| PBMCs mRNA expression | |||||
| CAT (%) | 100.0 ± 105.7 | 148.7 ± 35.0 | 112.4 ± 32.5 | 191.5 ± 113.8 * | 0.005 |
| MnSOD (%) | 100.0 ± 123.8 | 150.6 ± 124.1 | 131.5 ± 72.7 | 181.3 ± 109.2 | 0.665 |
| TLR4 (%) | 100.0 ± 87.9 | 76.5 ± 103.9 | 116.5 ± 91.7 | 58.7 ± 35.1 * | 0.024 |
| GPx (%) | 100.0 ± 176.6 | 120.7 ± 153.4 | 129.8 ± 162.5 | 156.1 ± 218.2 | 0.632 |
| GRd (%) | 100.0 ± 70.3 | 58.3 ± 51.3 | 86.9 ± 65.9 | 107.7 ± 90.8 | 0.132 |
| Oxidative damage | |||||
| MDA (ng/109 erythrocytes) | 1.6 ± 0.4 | 1.5 ± 0.4 | 1.6 ± 0.4 | 1.1 ± 0.2 *# | 0.003 |
| MDA (ng/L plasma) | 1.8 ± 0.6 | 0.1 ± 0.1 * | 1.9 ± 0.7 | 0.2 ± 0.1 * | 0.843 |
| oxLDL (ng/mL) | 34.0 ± 17.4 | 31.8 ± 8.7 | 35.4 ± 24.1 | 25.3 ± 13.5 * | 0.097 |
| ROS production in PBMCs stimulated with zymosan (RLU/min·103 cells) | 2199 ± 1757 | 1054 ± 476 | 2887 ± 1402 | 1022 ± 514 * | 0.481 |
| ROS production in PBMCs stimulated with LPS (RLU/min·103 cells) | 802.8 ± 1017 | 1702 ± 1960 | 451.4 ± 345.8 | 385.4 ± 329.8 * | 0.213 |
| p-Value | ||
|---|---|---|
| Anthropometry | ||
| Δ Weight (kg) | −0.638 | <0.001 |
| Δ BMI (kg/m2) | −0.629 | <0.001 |
| Δ Systolic BP (mmHg) | −0.163 | 0.336 |
| Δ Diastolic BP (mmHg) | −0.194 | 0.250 |
| Clinical parameters | ||
| Δ Glycemia (mg/dL) | −0.399 | 0.014 |
| Δ HbA1c (%) | −0.255 | 0.128 |
| Δ Cholesterol total (mg/dL) | −0.220 | 0.191 |
| Δ HDL-chol (mg/dL) | −0.053 | 0.756 |
| Δ LDL-chol (mg/dL) | −0.118 | 0.488 |
| Δ Triglycerides (mg/dL) | −0.146 | 0.389 |
| Δ CRP (mg/dL) | −0.032 | 0.852 |
| Δ AST (U/L) | −0.329 | 0.046 |
| Δ ALT (U/L) | −0.234 | 0.163 |
| Δ GGT (U/L) | −0.251 | 0.135 |
| Δ IFC (%) | −0.364 | 0.027 |
| Haematological parameters | ||
| Δ Haematocrit (%) | −0.019 | 0.242 |
| Δ Erythrocytes (106/μL) | −0.326 | 0.049 |
| Δ Leukocytes (103/μL) | 0.283 | 0.090 |
| Δ Platelets (103/μL) | 0.000 | 0.999 |
| Δ Neutrophils (103/μL) | 0.274 | 0.100 |
| Δ Lymphocytes (103/μL) | 0.010 | 0.952 |
| Δ Monocytes (103/μL) | 0.273 | 0.102 |
| Δ Eosinophils (103/μL) | 0.150 | 0.375 |
| Δ Basophils (103/μL) | 0.186 | 0.271 |
| Oxidative damage | ||
| Δ MDA (ng/109 erythrocytes) | −0.244 | 0.145 |
| Δ MDA (ng/L plasma) | −0.042 | 0.877 |
| b | SE b | b Standardized | |
|---|---|---|---|
| Constant | −0.504 | 1.924 | |
| Age | 0.045 | 0.037 | 0.165 |
| Sex | 0.033 | 0.444 | 0.009 |
| Intervention group | −0.342 | 0.311 | −0.148 |
| Δ Antioxidant Score | 0.238 | 0.046 | 0.659 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Argelich, E.; Casares, M.; Ugarriza, L.; Llompart, I.; Tur, J.A.; Sureda, A. Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study. Antioxidants 2024, 13, 480. https://doi.org/10.3390/antiox13040480
Quetglas-Llabrés MM, Monserrat-Mesquida M, Bouzas C, García S, Argelich E, Casares M, Ugarriza L, Llompart I, Tur JA, Sureda A. Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study. Antioxidants. 2024; 13(4):480. https://doi.org/10.3390/antiox13040480
Chicago/Turabian StyleQuetglas-Llabrés, Maria Magdalena, Margalida Monserrat-Mesquida, Cristina Bouzas, Silvia García, Emma Argelich, Miguel Casares, Lucía Ugarriza, Isabel Llompart, Josep A. Tur, and Antoni Sureda. 2024. "Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study" Antioxidants 13, no. 4: 480. https://doi.org/10.3390/antiox13040480
APA StyleQuetglas-Llabrés, M. M., Monserrat-Mesquida, M., Bouzas, C., García, S., Argelich, E., Casares, M., Ugarriza, L., Llompart, I., Tur, J. A., & Sureda, A. (2024). Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study. Antioxidants, 13(4), 480. https://doi.org/10.3390/antiox13040480