Natural Nanoparticles: A Particular Matter Inspired by Nature
Abstract
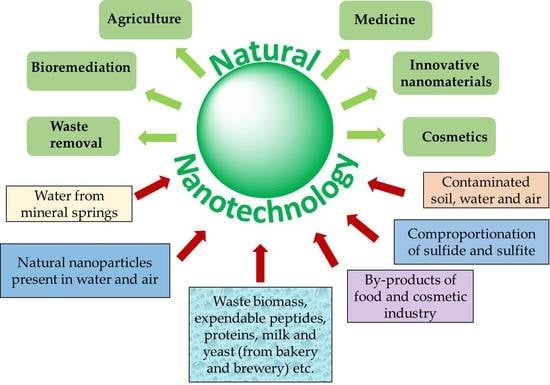
:1. Introduction
2. Natural but Not Biological: The Free Flow of Inorganic Nanocomposites
3. Bioreductive Formation of Nanoparticles
4. Redox Chemistry with Natural Products
5. Milling Vanilla
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kettler, K.; Krystek, P.; Giannakou, C.; Hendriks, A.J.; de Jong, W.H. Exploring the effect of silver nanoparticle size and medium composition on uptake into pulmonary epithelial 16HBE14o-cells. J. Nanopart. Res. 2016, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.M.; Siccardi, M. Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modelling. Br. J. Pharmacol. 2014, 171, 3963–3979. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.B.; Loeve, S. Metaphors in nanomedicine: The case of targeted drug delivery. NanoEthics 2014, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Roy, D.N.; Goswami, R.; Pal, A. Nanomaterial and toxicity: What can proteomics tell us about the nanotoxicology? Xenobiotica 2017, 47, 632–643. [Google Scholar]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef] [PubMed]
- Cassee, F.R.; Heroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.; Bozó, L.; Palmgren, F.; Sokhi, R.S. Particulate matter in urban air. In Air Quality in Cities: Saturn Eurotrac-2 Subproject Final Report; Moussiopoulos, N., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 91–120. [Google Scholar]
- Urbano, P.; Urbano, F. Nanobacteria: Facts or fancies? PLoS Pathog. 2007, 3, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Kajander, E.O.; Ciftcioglu, N.; Miller-Hjelle, M.A.; Hjelle, J.T. Nanobacteria: Controversial pathogens in nephrolithiasis and polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 2001, 10, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ciftcioglu, N.; Mckay, D.S.; Mathew, G.; Kajander, E.O. Nanobacteria: Fact or fiction? Characteristics, detection, and medical importance of novel self-replicating, calcifying nanoparticles. J. Investig. Med. 2006, 54, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pasula, R.R.; Lim, S. Engineering nanoparticle synthesis using microbial factories. In Engineering Biology; Institution of Engineering and Technology: Stevenage, UK, 2017; Volume 1, pp. 12–17. [Google Scholar]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Ramani, R.; Parischa, R.; Ajayakumar, P.V.; Alam, M.; et al. Bioreduction of AuCl4− ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Lahde, A.; Gudmundsdottir, S.S.; Joutsensaari, J.; Tapper, U.; Ruusunen, J.; Ihalainen, M.; Karhunen, T.; Torvela, T.; Jokiniemi, J.; Jarvinen, K.; et al. In vitro evaluation of pulmonary deposition of airborne volcanic ash. Atmos. Environ. 2013, 70, 18–27. [Google Scholar] [CrossRef]
- Murr, L.E.; Guerrero, P.A. Carbon nanotubes in wood soot. Atmos. Sci. Lett. 2006, 7, 93–95. [Google Scholar] [CrossRef]
- Wu, C.Y.; Martel, J.; Wong, T.Y.; Young, D.; Liu, C.C.; Lin, C.W.; Young, J.D. Formation and characteristics of biomimetic mineralo-organic particles in natural surface water. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Koo, A.N.; Min, K.H.; Lee, H.J.; Jegal, J.H.; Lee, J.W.; Lee, S.C. Calcium carbonate mineralized nanoparticles as an intracellular transporter of cytochromec for cancer therapy. Chem. Asian J. 2015, 10, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.H.; Wang, H.C.; Chung, R.S.; Hsu, J.C. Calcium carbonate nanoparticles can enhance plant nutrition and insect pest tolerance. J. Pestic. Sci. 2015, 40, 208–213. [Google Scholar] [CrossRef]
- Setiawan, H.; Khairani, R.; Rahman, M.A.; Septawendar, R.; Mukti, R.R.; Dipojono, H.K.; Purwasasmita, B.S. Synthesis of zeolite and gamma-alumina nanoparticles as ceramic membranes for desalination applications. J. Aust. Ceram. Soc. 2017, 53, 531–538. [Google Scholar] [CrossRef]
- Singh, I.B.; Gupta, A.; Dubey, S.; Shafeeq, M.; Banerjee, P.; Sinha, A.S.K. Sol-gel synthesis of nanoparticles of gamma alumina and their application in defluoridation of water. J. Sol-Gel Sci. Technol. 2016, 77, 416–422. [Google Scholar] [CrossRef]
- Nazari, A.; Sanjayan, J.G. Hybrid effects of alumina and silica nanoparticles on water absorption of geopolymers: Application of Taguchi approach. Measurement 2015, 60, 240–246. [Google Scholar] [CrossRef]
- Palaniraja, J.; Arunachalam, P.; Vijayalakshmi, U.; Ghanem, M.A.; Roopan, S.M. Synthesis of calcium silicate nanoparticles and its catalytic application in Friedlander reaction. Inorg. Nano-Met. Chem. 2017, 47, 946–949. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.J.; Chen, F.; Zhao, X.Y.; Zhao, J.; Qi, C. Amorphous calcium silicate hydrate/block copolymer hybrid nanoparticles: Synthesis and application as drug carriers. Dalton Trans. 2013, 42, 7032–7040. [Google Scholar] [CrossRef] [PubMed]
- Strambeanu, N.; Demetrovici, L.; Dragos, D. Natural sources of nanoparticles. In Nanoparticles’ Promises and Risks: Characterization, Manipulation, and Potential Hazards to Humanity and the Environment; Lungu, M., Neculae, A., Bunoiu, M., Biris, C., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 9–19. [Google Scholar]
- Sun, R.; Yin, L.; Zhang, S.H.; He, L.; Cheng, X.J.; Wang, A.N.; Xia, H.W.; Shi, H.B. Simple light-triggered fluorescent labeling of silica nanoparticles for cellular imaging applications. Chem. Eur. J. 2017, 23, 13893–13896. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.G.; Ma, X.Q.; Gao, Y.E.; Hou, M.L.; Xue, P.; Li, C.M.; Kang, Y.J. Multifunctional silica nanoparticles as a promising theranostic platform for biomedical applications. Mater. Chem. Front. 2017, 1, 1257–1272. [Google Scholar] [CrossRef]
- Liu, X.N.; Lu, X.R.; Wen, P.; Shu, X.Y.; Chi, F.T. Synthesis of ultrasmall silica nanoparticles for application as deep-ultraviolet antireflection coatings. Appl. Surf. Sci. 2017, 420, 180–185. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163–210. [Google Scholar] [CrossRef] [PubMed]
- Stawski, T.M.; Van Driessche, A.E.S.; Ossorio, M.; Rodriguez-Blanco, J.D.; Besselink, R.; Benning, L.G. Formation of calcium sulfate through the aggregation of sub-3 nanometre primary species. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Mohan, K.; Al-Sanousi, A.; Almaghrabi, B.; Genco, R.J.; Swihart, M.T.; Dziak, R. Synthesis and characterization of nanocrystalline calcium sulfate for use in osseous regeneration. Biomed. Mater. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Barnard, A.S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability. J. Mater. Chem. A 2013, 1, 27–42. [Google Scholar] [CrossRef]
- Groult, H.; Poupard, N.; Herranz, F.; Conforto, E.; Bridiau, N.; Sannier, F.; Bordenave, S.; Piot, J.M.; Ruiz-Cabello, J.; Fruitier-Arnaudin, I.; et al. Family of bioactive heparin-coated iron oxide nanoparticles with positive contrast in magnetic resonance imaging for specific biomedical applications. Biomacromolecules 2017, 18, 3156–3167. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Vahidi, O.; Bonakdar, S. Synthesis and characterization of glycyrrhizic acid coated iron oxide nanoparticles for hyperthermia applications. Mater. Sci. Eng. C 2017, 77, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Elrouby, M.; Abdel-Mawgoud, A.M.; Abd El-Rahman, R. Synthesis of iron oxides nanoparticles with very high saturation magnetization form TEA-Fe(III) complex via electrochemical deposition for supercapacitor applications. J. Mol. Struct. 2017, 1147, 84–95. [Google Scholar] [CrossRef]
- Perez, J.M. Iron oxide nanoparticles—Hidden talent. Nat. Nanotechnol. 2007, 2, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. Umber. Available online: https://en.wikipedia.org/wiki/Umber (accessed on 19 December 2017).
- Yuan, J.K.; Yang, J.; Suib, S.L. Synthesis of microporous manganese oxide nanoparticles and their catalysis applications. Abstr. Pap. Am. Chem. Soc. 2004, 227, U1310. [Google Scholar]
- Zhao, D.Y.; Han, B.; Xie, W.B.; An, B. Applications of stabilized manganese oxide and Fe-Mn binary oxides nanoparticles for in situ remediation of contaminated soil and groundwater. Abstr. Pap. Am. Chem. Soc. 2014, 247. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, J.; Li, J.C.; Yu, Z.B.; Zhang, G.X.; Shi, X.Y.; Shen, M.W. Facile synthesis and functionalization of manganese oxide nanoparticles for targeted T1-weighted tumor MR imaging. Colloid Surf. B 2015, 136, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Faulstich, L.; Griffin, S.; Nasim, M.J.; Masood, M.I.; Ali, W.; Alhamound, S.; Omran, Y.; Kim, H.; Kharma, A.; Schafer, K.H.; et al. Nature’s hat-trick: Can we use sulfur springs as ecological source for materials with agricultural and medical applications? Int. Biodeterior. Biodegrad. 2017, 119, 678–686. [Google Scholar] [CrossRef]
- Suleiman, M.; Al Ali, A.; Hussein, A.; Hammouti, B.; Hadda, T.B.; Warad, I. Sulfur nanoparticles: Synthesis, characterizations and their applications. J. Mater. Environ. Sci. 2013, 4, 1029–1033. [Google Scholar]
- Terrones, M. Carbon nanotubes: Synthesis and properties, electronic devices and other emerging applications. Int. Mater. Rev. 2004, 49, 325–377. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R 2004, 43, 61–102. [Google Scholar] [CrossRef]
- McGillicuddy, E.; Murray, I.; Kavanagh, S.; Morrison, L.; Fogarty, A.; Cormican, M.; Dockery, P.; Prendergast, M.; Rowan, N.; Morris, D. Silver nanoparticles in the environment: Sources, detection and ecotoxicology. Sci. Total Environ. 2017, 575, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Q.; Hu, Z.Q.; Deng, B.L. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Paralikar, P. Sulfur and sulfur nanoparticles as potential antimicrobials: From traditional medicine to nanomedicine. Expert Rev. Anti-Infect. 2016, 14, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Hough, R.M.; Noble, R.R.P.; Reich, M. Natural gold nanoparticles. Ore Geol. Rev. 2011, 42, 55–61. [Google Scholar] [CrossRef]
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar]
- Rauch, S.; Hemond, H.F.; Barbante, C.; Owari, M.; Morrison, G.M.; Peucker-Ehrenbrink, B.; Wass, U. Importance of automobile exhaust catalyst emissions for the deposition of platinum, palladium, and rhodium in the northern hemisphere. Environ. Sci. Technol. 2005, 39, 8156–8162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Liu, Y. Multifunctional platinum-based nanoparticles for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Sheny, D.S.; Philip, D.; Mathew, J. Synthesis of platinum nanoparticles using dried anacardium occidentale leaf and its catalytic and thermal applications. Spectrochim. Acta Part A 2013, 114, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Ortega, D.; Pankhurst, Q.A.; Thanh, N.T.K. Elucidating the morphological and structural evolution of iron oxide nanoparticles formed by sodium carbonate in aqueous medium. J. Mater. Chem. 2012, 22, 12498–12506. [Google Scholar] [CrossRef]
- Cho, M.H.; Choi, E.-S.; Kim, S.; Goh, S.-H.; Choi, Y. Redox-responsive manganese dioxide nanoparticles for enhanced MR imaging and radiotherapy of lung cancer. Front. Chem. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Q.; Rao, R.C.; Yang, H.X.; Liu, H.D.; Zhang, A.M. Facile synthesis of Fe3O4/MWCNTs by spontaneous redox and their catalytic performance. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. Integr. Environ. Assess. 2017, 13, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Lodyga-Chrucinska, E.; Chrustowicz, J. Fate of platinum metals in the environment. J. Trace Elem. Med. Biol. 2014, 28, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Sures, B. Significance of platinum group metals emitted from automobile exhaust gas converters for the biosphere. Environ. Sci. Pollut. Res. 2004, 11, 194–199. [Google Scholar] [CrossRef]
- Ezoe, Y.; Lin, C.H.; Noto, M.; Watanabe, Y.; Yoshimura, K. Evolution of water chemistry in natural acidic environments in Yangmingshan, Taiwan. J. Environ. Monit. 2002, 4, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Berlo, K.; van Hinsberg, V.J.; Vigouroux, N.; Gagnon, J.E.; Williams-Jones, A.E. Sulfide breakdown controls metal signature in volcanic gas at Kawah Ijen volcano, Indonesia. Chem. Geol. 2014, 371, 115–127. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Waring, R.H. Sulphate absorption across biological membranes. Xenobiotica 2016, 46, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. Redox signalling via the cellular thiolstat. Biochem. Soc. Trans. 2011, 39, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.; Nasim, M.; Ali, W.; Jacob, C. The reactive sulfur species concept: 15 years on. Antioxidants 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Cummins, L.M.; Kimura, E.T. Safety evaluation of selenium sulfide antidandruff shampoos. Toxicol. Appl. Pharmacol. 1971, 20, 89–96. [Google Scholar] [CrossRef]
- Evolution. Available online: https://en.Wikipedia.Org/wiki/evolution_(2001_film) (accessed on 20 December 2017).
- Schneider, T.; Baldauf, A.; Ba, L.A.; Jamier, V.; Khairan, K.; Sarakbi, M.B.; Reum, N.; Schneider, M.; Roseler, A.; Becker, K.; et al. Selective antimicrobial activity associated with sulfur nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Brown, T. The human genome. In Genomes, 2nd ed.; Wiley: Oxford, UK, 2002. [Google Scholar]
- Shors, T. Virus architecture and nomenclature. In Understanding Viruses, 2nd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2011. [Google Scholar]
- Janssen, A.; de Keizer, A.; van Aelst, A.; Fokkink, R.; Yangling, H.; Lettinga, G. Surface characteristics and aggregation of microbiologically produced sulphur particles in relation to the process conditions. Colloid Surf. B 1996, 6, 115–129. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, B.R.; Naqvi, A.H.; Singh, H.B. Potential of biosynthesized silver nanoparticles using Stenotrophomonas sp. BHU-S7 (MTCC 5978) for management of soil-borne and foliar phytopathogens. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, B.R.; Singh, A.; Keswani, C.; Naqvi, A.H.; Singh, H.B. Biofabricated silver nanoparticles act as a strong fungicide against Bipolaris sorokiniana causing spot blotch disease in wheat. PLoS ONE 2014, 9, e97881. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Dolma, K.; Kaur, N.; Rathore, Y.S.; Ashish; Mayilraj, S.; Choudhury, A.R. Biosynthesis of gold and silver nanoparticles using a novel marine strain of Stenotrophomonas. Bioresour. Technol. 2013, 142, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kundu, S. Biosynthesis of gold nanoparticles using bacteria. Proc. Natl. Acad. Sci. India Sect. B 2014, 84, 331–336. [Google Scholar] [CrossRef]
- Stefess, G.C.; Torremans, R.A.M.; DeSchrijver, R.; Robertson, L.A.; Kuenen, J.G. Quantitative measurement of sulphur formation by steady state and transient state continuous cultures of autotrophic thiobacillus species. Appl. Microbiol. Biot. 1996, 45, 169–175. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.H.; Farooqui, S.M.; White, A.R.; Greene, A.C. Production of manganese oxide nanoparticles by Shewanella species. Appl. Environ. Microbiol. 2016, 82, 5402–5409. [Google Scholar] [CrossRef] [PubMed]
- Garmasheva, I.; Kovalenko, N.; Voychuk, S.; Ostapchuk, A.; Livins’ka, O.; Oleschenko, L. Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro. Bioimpacts 2016, 6, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.K.; Singh, J.K.; Gupta, N.; Kumar, V. A review of nanobioremediation technologies for environmental cleanup: A novel biological approach. J. Mater. Environ. Sci. 2017, 8, 740–757. [Google Scholar]
- Sanghi, R.; Verma, P.; Puri, S. Enzymatic formation of gold nanoparticles using phanerochaete chrysosporium. Adv. Chem. Eng. Sci. 2011, 1, 1–8. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Q.; Xu, H.Z.; Chen, Z.S.; Chen, G.F. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Estevam, E.C.; Griffin, S.; Nasim, M.J.; Denezhkin, P.; Schneider, R.; Lilischkis, R.; Dominguez-Alvarez, E.; Witek, K.; Latacz, G.; Keck, C.; et al. Natural selenium particles from Staphylococcus carnosus: Hazards or particles with particular promise? J. Hazard. Mater. 2017, 324, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.P.; Gao, P. Expulsion of selenium/protein nanoparticles through vesicle-like structures by saccharomyces cerevisiae under microaerophilic environment. World J. Microbiol. Biotechnol. 2012, 28, 3381–3386. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Yang, L.B.; Zhang, B.C.; Liu, J.H. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloid Surf. B 2010, 80, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Mahdavi, M.; Varastehmoradi, B.; Faramarzi, M.A.; Shahverdi, A.R. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: An in vivo study. Biol. Trace Elem. Res. 2012, 149, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Prakash, N.T.; Sharma, N.; Prakash, R.; Raina, K.K.; Fellowes, J.; Pearce, C.I.; Lloyd, J.R.; Pattrick, R.A.D. Aerobic microbial manufacture of nanoscale selenium: Exploiting nature’s bio-nanomineralization potential. Biotechnol. Lett. 2009, 31, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Manikova, D.; Letavayova, L.M.; Vlasakova, D.; Kosik, P.; Estevam, E.C.; Nasim, M.J.; Gruhlke, M.; Slusarenko, A.; Burkholz, T.; Jacob, C.; et al. Intracellular diagnostics: Hunting for the mode of action of redox-modulating selenium compounds in selected model systems. Molecules 2014, 19, 12258–12279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellucci Estevam, E.; Witek, K.; Faulstich, L.; Nasim, M.J.; Latacz, G.; Dominguez-Alvarez, E.; Kiec-Kononowicz, K.; Demasi, M.; Handzlik, J.; Jacob, C. Aspects of a distinct cytotoxicity of selenium salts and organic selenides in living cells with possible implications for drug design. Molecules 2015, 20, 13894–13912. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.A.; Mathew, Z. Bioremediation potential of white rot fungi. In British Mycological Society Symposium Series; Cambridge University Press: Cambridge, UK, 2001; pp. 52–78. [Google Scholar]
- Padhi, S.K.; Tripathy, S.; Sen, R.; Mahapatra, A.S.; Mohanty, S.; Maiti, N.K. Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int. Biodeterior. Biodegrad. 2013, 78, 67–73. [Google Scholar] [CrossRef]
- Duran, N.; Cuevas, R.; Cordi, L.; Rubilar, O.; Diez, M.C. Biogenic silver nanoparticles associated with silver chloride nanoparticles (Ag@AgCl) produced by laccase from Trametes versicolor. SpringerPlus 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Anil Kumar, S.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Singh, A.K.; Kanchanapally, R.; Fan, Z.; Senapati, D.; Ray, P.C. Synthesis of highly fluorescent water-soluble silver nanoparticles for selective detection of Pb(II) at the parts per quadrillion (PPQ) level. Chem. Commun. 2012, 48, 9047–9049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Rong, M.; Cai, Z.; Yang, C.J.; Chen, X. Sonochemical synthesis of highly fluorescent glutathione-stabilized Ag nanoclusters and S2− sensing. Nanoscale 2012, 4, 4103–4106. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mehata, M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chem. Eur. J. 2007, 13, 3160–3168. [Google Scholar] [CrossRef] [PubMed]
- Brodin, J.D.; Carr, J.R.; Sontz, P.A.; Tezcan, F.A. Exceptionally stable, redox-active supramolecular protein assemblies with emergent properties. Proc. Natl. Acad. Sci. USA 2014, 111, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Park, S.H.; Govarthanan, M.; Hwang, P.H.; Seo, Y.S.; Cho, M.; Lee, W.H.; Lee, J.Y.; Kamala-Kannan, S.; Oh, B.T. Synthesis of silver nanoparticles using cow milk and their antifungal activity against phytopathogens. Mater. Lett. 2013, 105, 128–131. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Yamada, C.; Kawai, H.; Yoshida, K. Improving ascorbic acid content of tomato fruits by the oxidized yeast extract. In Plant Nutrition for Sustainable Food Production and Environment, Proceedings of the XIII International Plant Nutrition Colloquium, Tokyo, Japan, 13–19 September 1997; Ando, T., Fujita, K., Mae, T., Matsumoto, H., Mori, S., Sekiya, J., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 973–974. [Google Scholar]
- Balcerczyk, A.; Grzelak, A.; Janaszewska, A.; Jakubowski, W.; Koziol, S.; Marszalek, M.; Rychlik, B.; Soszynski, M.; Bilinski, T.; Bartosz, G. Thiols as major determinants of the total antioxidant capacity (reprinted from thiol metabolism and redox regulation of cellular functions). Biofactors 2003, 17, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Capone, D.L.; Pardon, K.H.; Elsey, G.M.; Sefton, M.A.; Francis, I.L.; Pretorius, I.S. Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast 2007, 24, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.D.; Schulz, B.L. Vegemite beer: Yeast extract spreads as nutrient supplements to promote fermentation. PeerJ 2016, 4, e2271. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I.; Walker, S.E.; Mackenzie, S.; Knowles, S. Dietary restriction, tyramine, and the use of monoamine-oxidase inhibitors. J. Clin. Psychopharmacol. 1989, 9, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Musarrat, J.; Dwivedi, S.; Singh, B.R.; Al-Khedhairy, A.A.; Azam, A.; Naqvi, A. Production of antimicrobial silver nanoparticles in water extracts of the fungus Amylomyces rouxii strain KSU-09. Bioresour. Technol. 2010, 101, 8772–8776. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, T.V.; Ravikumar, S.; Eom, I.Y. Green synthesized silver nanoparticles using nelumbonucifera root extract for efficient protein binding, antioxidant and cytotoxicity activities. J. Photochem. Photobiol. B 2014, 141, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Sreekanth, T.V.M.; Eom, I.Y. Interaction studies of greenly synthesized gold nanoparticles with bovine serum albumin (BSA) using fluorescence spectroscopy. J. Nanosci. Nanotechnol. 2015, 15, 9617–9623. [Google Scholar] [CrossRef] [PubMed]
- Dhayalan, M.; Denison, M.I.J.; Jegadeeshwari, L.A.; Krishnan, K.; Gandhi, N.N. In vitro antioxidant, antimicrobial, cytotoxic potential of gold and silver nanoparticles prepared using Embelia ribes. Nat. Prod. Res. 2017, 31, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.; Popa, A.; Toloman, D.; Silipas, T.D.; Vodnar, D.C. Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium Sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Metall Sin. Engl. 2016, 29, 228–236. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Silipas, T.-D.; Vodnar, D.C.; Katona, G. Enhanced antibacterial activity of zinc oxide nanoparticles synthesized using Petroselinum crispum extracts. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2015; Volume 1700. [Google Scholar]
- Stan, M.; Lung, I.; Soran, M.L.; Leostean, C.; Popa, A.; Stefan, M.; Lazar, M.D.; Opris, O.; Silipas, T.D.; Porav, A.S. Removal of antibiotics from aqueous solutions by green synthesized magnetite nanoparticles with selected agro-waste extracts. Process Saf. Environ. 2017, 107, 357–372. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloid Surf. B 2013, 108, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta Part A 2012, 93, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Otunola, G.A.; Afolayan, A.J.; Ajayi, E.O.; Odeyemi, S.W. Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of Allium sativum, Zingiber officinale, and Capsicum frutescens. Pharmacogn. Mag. 2017, 13, S201–S208. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Chokshi, K.; Pancha, I.; Ghosh, T.; Paliwal, C.; Maurya, R.; Ghosh, A.; Mishra, S. Green synthesis, characterization and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae acutodesmus dimorphus. RSC Adv. 2016, 6, 72269–72274. [Google Scholar] [CrossRef]
- Harish, S.P. Phyco-nanotechnology: New horizons of gold nano-factories. Proc. Natl. Acad. Sci. India Sect. B 2016. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathy, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem 2009, 19, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.; Tittikpina, N.K.; Al-Marby, A.; Alkhayer, R.; Denezhkin, P.; Witek, K.; Gbogbo, K.A.; Batawila, K.; Duval, R.E.; Nasim, M.J.; et al. Turning waste into value: Nanosized natural plant materials of Solanum incanum L. and Pterocarpus erinaceus poir with promising antimicrobial activities. Pharmaceutics 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Development of an oral rutin nanocrystal formulation. Int. J. Pharm. 2009, 370, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.H.; Keck, C.M. Second generation of drug nanocrystals for delivery of poorly soluble drugs: smartCrystal technology. Eur. J. Pharm. Sci. 2008, 34, S20–S21. [Google Scholar] [CrossRef]
- Keck, C.M.; Muller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Keck, C.M. Twenty years of drug nanocrystals: Where are we, and where do we go? Eur. J. Pharm. Biopharm. 2012, 80, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Keck, C.M. Nanocrystals: From raw material to the final formulated oral dosage form—A review. Curr. Pharm. Des. 2015, 21, 4217–4228. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.; Alkhayer, R.; Mirzoyan, S.; Turabyan, A.; Zucca, P.; Sarfraz, M.; Nasim, M.; Trchounian, A.; Rescigno, A.; Keck, C.; et al. Nanosizing Cynomorium: Thumbs up for potential antifungal applications. Inventions 2017, 2. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Alihosseini, F.; Ghaffari, S.; Dabirsiaghi, A.R.; Haghighat, S. Freeze-drying of ampicillin solid lipid nanoparticles using mannitol as cryoprotectant. Braz. J. Pharm. Sci. 2015, 51, 797–802. [Google Scholar] [CrossRef]
- Patrignani, F.; Lanciotti, R. Applications of high and ultra high pressure homogenization for food safety. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia. Milli Vanilli. Available online: https://en.wikipedia.org/wiki/Milli_Vanilli (accessed on 20 December 2017).
- Ganesh Kumar, C.; Poornachandra, Y.; Mamidyala, S.K. Green synthesis of bacterial gold nanoparticles conjugated to resveratrol as delivery vehicles. Colloids Surf. B 2014, 123, 311–317. [Google Scholar] [CrossRef] [PubMed]








| Name | Chemical Formulae and Symbols | Natural Occurrence | Practical Implications (of Similar, Refined Materials) |
|---|---|---|---|
| Calcium Carbonate | CaCO3 | natural surface water [16] | industry, biotechnology, cancer therapy, drug delivery, plant nutrition and promotion of plant defense against pests [17,18,19,20] |
| Alumina | Al2O3 | desalination and defluorination of water [21,22,23] | |
| Silicate | SiO44− | drug carrier and catalytic applications [24,25] | |
| Silica | SiO2 | volcanic eruptions [26] | food additive, anti-caking agent, ultraviolet antireflection coating, cellular imaging and biomedical applications [27,28,29,30] |
| Bassanite (Calcium Sulfate) | CaSO4 | sea water [31] | bone regeneration [32] |
| Iron Oxide | Fe3O4 | iceberg-hosted sediments [33] | medical diagnostics, controlled drug release, hyperthermia, biosensors, supercapacitor applications [34,35,36,37] |
| Manganese oxide | MnO2 | umber [38] | imaging, remediation of contaminated soil and ground water, catalysis [39,40,41] |
| Sulfur | S | mineral wells [42] | medical applications, (antimicrobial, cytotoxic), fertilizers, fiber industry [43] |
| Soot (in the form of carbon) | C | atmospheric particulate matter | composite reinforcements, nano-reactors, chemical sensors, gas adsorbents, catalyst supports, templates, actuators, probes, nano-pipes [44,45] |
| Silver | Ag | aquatic environment [46,47] | antimicrobial properties, nano-functionalized plastics, paints, food containers, domestic appliances, textiles, medical products and cosmetics [46,48] |
| Gold | Au | ore deposits [49] | biosensorics, immunoassays, medical applications and laser phototherapy of tumors [50] |
| Platinum | Pt | automobile exhausts [51] | biomedical applications, nano-biomedicine, catalytic and thermal applications [52,53,54] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffin, S.; Masood, M.I.; Nasim, M.J.; Sarfraz, M.; Ebokaiwe, A.P.; Schäfer, K.-H.; Keck, C.M.; Jacob, C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants 2018, 7, 3. https://doi.org/10.3390/antiox7010003
Griffin S, Masood MI, Nasim MJ, Sarfraz M, Ebokaiwe AP, Schäfer K-H, Keck CM, Jacob C. Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants. 2018; 7(1):3. https://doi.org/10.3390/antiox7010003
Chicago/Turabian StyleGriffin, Sharoon, Muhammad Irfan Masood, Muhammad Jawad Nasim, Muhammad Sarfraz, Azubuike Peter Ebokaiwe, Karl-Herbert Schäfer, Cornelia M. Keck, and Claus Jacob. 2018. "Natural Nanoparticles: A Particular Matter Inspired by Nature" Antioxidants 7, no. 1: 3. https://doi.org/10.3390/antiox7010003
APA StyleGriffin, S., Masood, M. I., Nasim, M. J., Sarfraz, M., Ebokaiwe, A. P., Schäfer, K. -H., Keck, C. M., & Jacob, C. (2018). Natural Nanoparticles: A Particular Matter Inspired by Nature. Antioxidants, 7(1), 3. https://doi.org/10.3390/antiox7010003