1. Introduction
The agrimony herb is a traditional plant drug, which is commonly used as an astringent agent, as well as medication for inflammatory, cardiovascular, and other oxidative-related diseases [
1]. The genus
Agrimonia L. (
Rosaceae, Rosoideae) comprises 16 species of perennial herbs distributed in the northern hemisphere [
1].
Agrimonia procera Wallr., commonly known as fragrant agrimony, is a well-distinguished taxon growing in similar habitats to
A. eupatoria and is usually considered to be less common [
2]. According to European Pharmacopoeia, the only source of this plant drug is
Agrimonia eupatoria. By contrast, the German Commission E pharmacopoeial monograph is employed to allow
Agrimonia procera to be used as a second valid source of
Agrimoniae herba. Different effects were shown for
Agrimonia spp.: for example,
Agrimonia pilosa Ledeb. possesses several physiological activities, such as anti-cancer, antioxidant, anti-inflammatory, hepatoprotective, anti-Alzheimer, and anti-diabetic properties. The data on the phytochemistry of
A. procera are scarce [
1]. Pukalskienė et al. emphasized that the most important medicinal application of
A. procera might be protection of the cardiovascular system against menopause-induced changes [
3]. Gräber et al. showed that low dosages of
Agrimonia procera provide beneficial effects on feed intake in piglets [
4] and exert antimicrobial effects, as well as increase the immune response, in lipopolysaccharide-challenged piglets [
5].
Several studies have been conducted on the phytochemical composition of
Agrimonia spp.: Polyphenols are major constituents, including phenolic acids, flavonoids, ellagitannins, and procyanidins. These compounds are strong radical scavengers and may be responsible for the comparatively high antioxidant potential of this plant. Agrimoniin is reported as a major ellagitannin occurring in common agrimony in high quantities. Phytochemical investigations of
A. procera are scarce, but it was shown that this agrimony species contains agrimoniin, and flavonoids generally defined as luteolin and apigenin glycosides [
1,
3]. It is suggested that these proposed beneficial health effects are attributable to these phytochemicals [
1,
3].
A wide spectrum of pharmacological effects of
Agrimonia spp. has been reported, but mechanistic
in vivo reports about the antioxidative, antidiabetic, and anti-Alzheimer effects of the extract, as well as studies showing protection against stress, are limited [
1]. Therefore, we analyzed the effects of an ethanolic
Agrimonia procera extract (eAE) in the model organism
C. elegans. In our study, we focused on antioxidant effects
in vivo, stress resistance, protection against amyloid-β, and life span modulating effects. These results are important for the identification of molecular targets of eAE in humans (pharmacological use).
2. Materials and Methods
Materials: Trolox was purchased from Calbiochem (Merck, Darmstadt, Germany) and SYTOX
® Green Nucleic Acid Stain was obtained from Molecular Probes Inc. (Leiden, The Netherlands). All other chemicals were of analytical grade and were purchased from SIGMA-Aldrich (Taufkirchen, Germany).
Agrimonia procera “Magna” (harvested: June 2017) was provided by Exsemine GmbH (Salzatal, Germany), and the eAE was obtained by extraction of the dried plant material (
Agrimonia procera herba, polyphenol content: 5.54%, agrimoniin: 1.91%,
Figure 1A) using 100% ethanol (reflux, 1 h). The ethanolic extract was vaporized and the residue was completely dissolved in DMSO (stock solution: 250 mg/mL). In each experiment, the same amount of the vehicle (DMSO, 0.4%) was used.
C. elegans strains and maintenance: C. elegans strains used in this study (wild-type N2 var. Bristol, CF1038 [daf-16(mu86) I.], TJ356 [zIs356 IV (daf-16p::daf-16a/b::GFP+rol-6)], CL4176 [smg-1(cc546) I; dvls27 X.]) and the bacterial strains were provided by the Caenorhabditis Genetics Center (CGC). Strain maintenance was performed at 20 °C on nematode growth medium (NGM) agar plates containing a lawn of E. coli var. OP50 as the food source. Compound treatment of C. elegans was conducted in 1.5 mL of liquid NGM containing 1% (w/v) bovine serum albumin, 12.5 µg/mL tetracycline, 100 µg/mL ampicillin, and E. coli OP50-1 (109 cfu/mL) in 35 mm petri dishes. Stock solution (250 mg/mL) of eAE was prepared in DMSO and concentrations of 50, 100, and 200 µg/mL eAE were used for each experiment. Age synchronous nematodes were obtained by treating gravid adults with a bleaching solution (50% NaOH (5 M)/50% NaClO (13%)), followed by three washing steps in liquid NGM. The remaining eggs were allowed to hatch on fresh NGM agar plates seeded with OP50. After three days at 20 °C, an age synchronous population of L4 larvae and young adult animals were used for experiments. For the paraquat assay, an age synchronous population was generated by timed egg-laying: Gravid adult nematodes were transferred on NGM agar plates containing a lawn of E. coli var. OP50 and were allowed to lay eggs. After 2 h, all adult animals were removed from the plate and the eggs were incubated for three days at 20 °C. For the Aβ assay, synchronization was performed by egg laying in liquid NGM media with E. coli OP50-1 (109 cfu/mL).
Antioxidative capacity in vitro (TEAC assay): The trolox equivalent antioxidative capacity (TEAC) assay is a cell-free method for the detection of radical-scavenging properties of test compounds in comparison to the synthetic vitamin E derivative trolox (positive control). The ABTS stock solution (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), a stable colored radical) was stored in the dark overnight and subsequently diluted with ethanol to obtain an absorption of 0.6 at 734 nm. Aliquots (10 µL) of eAE or trolox in various concentrations were pipetted into the wells of a transparent 96-well microtiter plate, mixed with 290 µL of the radical solution, and stored at room temperature for 12 min to start the reaction. The absorption was measured spectrophotometrically at 734 nm.
Measurement of intracellular ROS accumulation in vivo: Synchronized L4 larvae and young adults (N2 and CF1038) were treated with different eAE concentrations for 24 h, washed in phosphate buffered saline/0.1% Tween 20 (PBST), and transferred individually in 1 µL PBST into the wells of a 384-well microtiter plate. The wells contained 7 µL M9 buffer. After the transfer of all nematodes, 2 μL of 250 μM H2DCF-DA was added to each well and the plate was sealed to prevent evaporation. During thermal stress (37 °C), fluorescence intensities (excitation: 485 nm; emission: 535 nm) were recorded with a fluorescence reader. Fluorescence values were normalized to the increase of the control value (t = 7 h).
Oxidative stress resistance (Paraquat assay): Synchronized L4 larvae and young adults were treated for three days, with different concentrations of eAE or with DMSO as a control and with FUDR (5-fluorodeoxyuridine) to prevent progeny from hatching. Then, the nematodes were transferred into eAE-free medium containing 50 mM paraquat. For the following four days, the survival of the nematodes was analyzed by touch-provoked movement every 24 h. This experiment was carried out with wild-type nematodes and with the loss-of-function mutant strain CF1038.
Thermal stress resistance (SYTOX
® Green assay): Synchronized L4 larvae and young adult animals (N2 and CF1038) were treated with eAE (see above) for 24 h. Prior to the application of thermal stress, animals were washed in PBST. After transferring each nematode in 1 µL of PBST into a well of a 384-well microtiter plate containing 9 µL PBS, SYTOX
® Green Nucleic Acid Stain in PBS (10 μL of a 2 μM solution) was added and the wells were sealed to avoid evaporation. Thermal stress (37 °C) was applied and the increase of fluorescence which correlates with an increase in cells with disturbed membrane integrity was measured (excitation: 485 nm; emission: 535 nm) with a fluorescence reader. When the fluorescence values of individual nematodes exceeded a defined cut-off value (mean fluorescence values of the first four measurements multiplied by the factor three), the corresponding animal was scored as dead. The description of the death criteria was adopted from Gill et al. (2003) [
6] and verified by manual analysis (touch-provoked movement).
Life span: For the analysis of the life span at 25 °C, the wild-type strain N2, as well as CF1038, was used. Forty synchronized L4 larvae and young adult animals were transferred into liquid media (as described above). This time point was considered as day 0 of the life span. During the first 10 days of the life span, the media contained 120 µM FUDR to prevent the hatching of viable progeny. The incubation media were exchanged every day and the survival of the animals was measured by touch-provoked movement.
Aβ Assay: Eggs of the transgenic strain CL4176 were incubated with different concentrations of eAE or DMSO as a control or caffeine (5 mM, [
7]) as a positive control for two days. L3 larvae were transferred from the media onto agar plates containing a lawn of
E. coli var. OP50 and stored at 25 °C. After 26, 28, 30, 32, and 34 h, all nematodes were tested by touch-provoked movement. Nematodes only slightly moving their head or not moving at all were scored as paralyzed.
Intracellular localization of DAF-16::GFP: The transgenic strain TJ356 was used to detect the intracellular localization of the GFP-tagged transcription factor DAF-16. Synchronized L4 larvae and young adult animals of the corresponding strains were transferred into liquid treatment media (as described above) and maintained for one hour at 20 °C, respectively. Subsequently, a drop of 9 µL medium containing the nematodes was placed on a microscope slide and mixed with 9 µL of the anesthetic levamisole (20 mM). After the application of a cover slip, the cellular localization of DAF-16::GFP in the whole body was detected by fluorescence microscopy.
Statistics: Statistical analyses were conducted for at least three independent experiments, with data given as mean ± SD. PASW Statistics for Windows, Version 18.0 (SPSS Inc.; Chicago, IL, USA) and GraphPad Prism 6 (La Jolla, CA, USA) software was used to compute the statistical analyses. Statistical significance was determined by one-way or two-way Analysis of variance (ANOVA) with Dunnett’s or Tukey’s post hoc-test, while life span, oxidative, and thermal stress resistance, as well as Aβ paralysis curves, were calculated using Kaplan-Meier survival analyses with a log-rank test. Statistical differences were considered to be significant at a level of p ≤ 0.05.
4. Discussion
It is well-known that extracts from
Agrimonia spp. possess a high radical scavenging capacity, which is measured via the reduction of colored radicals (ABTS: 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid); DPPH: 2,2-Diphenyl-1-picrylhydrazyl)
in vitro [
3,
9]. It is also known that extracts from
Agrimonia spp. are able to protect against oxidative stress
in vivo [
9]. The aim of our study was to investigate the antioxidative effects of an agrimony extract in greater detail to show, e.g., dose-dependent effects
in vivo, as well as molecular mechanisms of the effects using the model organism
Caenorhabditis elegans. Antioxidative effects of plant compounds/plant extracts
in vivo may be directly mediated via radical scavenging effects mediated by phenolic groups (as detectable in the TEAC assay). On the other hand, plant compounds/plant extracts may also cause indirect antioxidative effects via an interference with intracellular signaling processes [
10,
11]. These indirect effects will result in an increased expression of antioxidative enzymes like superoxide dismutase or catalase. A pivotal transcription factor for the expression of antioxidative enzymes is the DAF-16 transcription factor, a downstream component of the insulin-like signaling pathway [
12,
13]. As an orthologue of mammalian FoxO proteins, DAF-16 is inactivated after insulin signaling via a shuttle from the nucleus to the cytosol. This key transcription factor integrates different signals from the insulin/IGF-1 signaling pathway, mammalian target of rapamycin (mTOR) signaling, AMP-activated protein kinase (AMPK) pathway, c-Jun N-terminal kinase (JNK) pathway, and germline signaling to modulate aging and longevity [
13].
In our model system, we were able to show both a protection against oxidative stress (paraquat) and a decrease in ROS formation
in vivo (DCF assay). However, this decrease in ROS formation was not mediated by a modulation of DAF-16 since antioxidative effects were still detectable in DAF-16 loss-of-function animals. The reduction of ROS caused by eAE may be mediated by the strong radical scavenging effect of the constituents of the extract. Other authors reported an interference of agrimony compounds with specific components of various cellular signal transduction pathways. For example, Wang et al. showed that agrimol B, a polyphenol derived from
Agrimonia pilosa Ledeb., significantly induced cytoplasm-to-nucleus shuttle of SIRT1, a histone deacetylase [
14]. Since this enzyme is involved in regulating the life span of organisms, we further analyzed the effect of eAE on the life span of
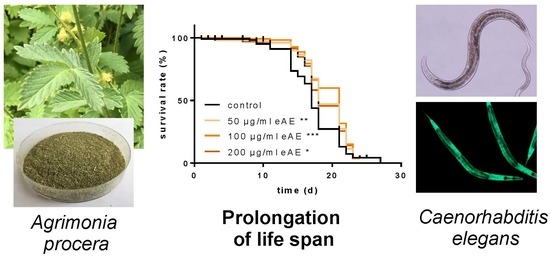
C. elegans. In our study, we were the first to show that this plant is able to promote longevity: the high concentrations tested (100 and 200 µg/mL) increased the life span of the nematodes. Additionally, we were able to show that this effect is strongly dependent on the transcription factor DAF-16, since in nematodes without this factor (loss-of-function animals), no prolongation of life span was detectable. Even more, eAE resulted in a decrease in life span of these loss-of-function animals. We conclude that this transcription factor is important in mediating the effects of eAE. Since it is known that DAF-16 has pleiotropic functions in the nematode, we analyzed whether it is necessary for the other effects reported in our study. This was the case: both the protection against thermal stress and oxidative stress was completely dependent on DAF-16. Since this factor is also a component of the insulin-like signaling pathway, eAE may be interesting for the treatment of diabetes and related metabolic diseases.
This connection is not new: A first correlation between agrimony and diabetes was reported in 1990 by Swanston-Flatt et al. [
15]. They showed that agrimony is able to reduce the level of hyperglycaemia during the development of streptozotocin diabetes in mice, at least mediated via antioxidant effects [
15]. The involvement of distinct bioactive compounds from
Agrimonia spp. and insulin resistance was shown more recently by Teng et al. They demonstrated that agrimonolide and desmethylagrimonolide isolated from
Agrimonia pilosa Ledeb. were able to modulate the glucose metabolism in insulin-resistant HepG2 cells: both compounds elevated glucokinase activity, reduced glucose-6-phosphatase activity, and increased the insulin-mediated glycogen level in hepatocytes [
16]. Wang et al. demonstrated that agrimol inhibited the differentiation of the adipocytes, as well as adipogenesis [
14]. Beneficial effects of an aqueous
Agrimonia extract were also reported in an animal model showing that this extract improves the impaired glucose tolerance in high-fat diet-fed rats by decreasing the inflammatory response [
17].
In our experimental model system, we were able to show that the occurrence of a transcription factor downstream of the insulin signaling pathway is essential for mediating the beneficial effects of eAE in the nematode. However, we were not able to show the interference between eAE and the DAF-16 transcription factor directly (no significant change in intracellular localization after incubation with eAE), but maybe the incubation time (1 h) was too short.
Another point which was not observed in our experimental system was protection against amyloid-β-toxicity. Since
Agrimonia spp. are rich in secondary plant compounds, it has been suggested that this plant should protect against neurodegenerative diseases like Alzheimer’s disease. It was shown that distinct phytochemicals isolated from
Agrimonia pilosa Ledeb. were able to inhibit the acetylcholine esterase [
18,
19]. Kubinova et al. showed that an extract of
A. procera (100 μg/mL) also displayed the inhibition of acetylcholine esterase [
20]. Lee et al. reported that an extract of
Agrimonia eupatoria attenuated glutamate-induced oxidative stress in hippocampal cells [
21]. Park et al. demonstrated that water extracts of
Agrimonia pilosa Ledeb. prevented amyloid-β (25-35)-induced cell death in PC12 cells. Furthermore, the extract improved cognitive dysfunction and glucose homeostasis in rats with experimentally-induced AD-type dementia (hippocampal infusions of plaque-forming amyloid-β (25-35) or non-plaque forming amyloid-β (35-25)) [
22]. We used
Caenorhabditis elegans as a simple model to evaluate the protective effects of eAE against amyloid-β-toxicity. However, in this model, no protection against amyloid-β-toxicity was detectable (up to 200 µg/mL), while caffeine as a positive control strongly protected the animals from the toxic amyloid stress. The discrepancy between the nematode and rat may be due to the simplified model, since only a specific amyloid-β-form is expressed. However, caffeine as a positive control resulted in strong protection; furthermore, acetylcholine esterase inhibitors are able to cause protection in our experimental system (aldicarb, donepezil; data not shown). Maybe the amount of phytochemicals with acetylcholinesterase-inhibiting properties was too low in the extract concentration used (200 µg/mL).