Microbial Biotransformation of a Polyphenol-Rich Potato Extract Affects Antioxidant Capacity in a Simulated Gastrointestinal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenolic Extraction
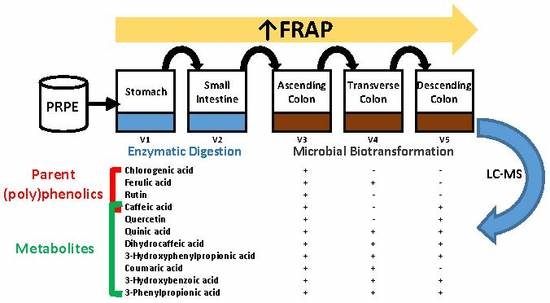
2.2. In Vitro Digestion of PRPE
2.3. LC-MS Analysis of Phenolic Metabolites
2.4. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Biotransformation of Polyphenols
3.2. Antioxidant Capacity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Solayman, M.; Ali, Y.; Alam, F.; Islam, M.A.; Alam, N.; Khalil, M.I.; Gan, S.H. Polyphenols: Potential future arsenals in the treatment of diabetes. Curr. Pharm. Des. 2016, 22, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zarrelli, A.; Sgambato, A.; Petito, V.; De Napoli, L.; Previtera, L.; Di Fabio, G. New C-23 modified of silybin and 2,3-dehydrosilybin: Synthesis and preliminary evaluation of antioxidant properties. Bioorg. Med. Chem. Lett. 2011, 21, 4389–4392. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Hale, A.L.; Miller, J.C. Determination of phenolic content, composition and their contribution to antioxidant activity in specialty potato selections. Am. J. Potato Res. 2007, 84, 275–282. [Google Scholar] [CrossRef]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and human health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, V.; Di Fabio, G.; Di Marino, C.; Davinelli, S.; Scapagnini, G.; Zarrelli, A. Evaluation of new strategies to reduce the total content of α-solanine and α-chaconine in potatoes. Phytochem. Lett. 2018, 23, 116–119. [Google Scholar] [CrossRef]
- Romanucci, V.; Pisanti, A.; Di Fabio, G.; Davinelli, S.; Scapagnini, G.; Guaragna, A.; Zarrelli, A. Toxin levels in different variety of potatoes: Alarming contents of α-chaconine. Phytochem. Lett. 2016, 16, 103–107. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.O.; Smith, N.; Schroeder, D.; Han, J.T.; Lee, C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food Agric. 2005, 85, 1715–1724. [Google Scholar] [CrossRef]
- Brat, P.; George, S.; Bellamy, S.; Du Chaffaut, L.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Kubow, S.; Hobson, L.; Iskandar, M.M.; Sabally, K.; Donnelly, D.J.; Agellon, L.B. Extract of Irish potatoes (Solanum tuberosum L.) decreases body weight gain and adiposity and improves glucose control in the mouse model of diet-induced obesity. Mol. Nutr. Food Res. 2014, 58, 2235–2238. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; de Vries, J.H.M.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Buijsman, M.N.; van Amelsvoort, J.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 510, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Xu, A.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; Seeram, N.P.; Heber, D.; et al. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Bolca, S.; Verstraete, W.; Heyerick, A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 2011, 82, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analysing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Guy, P.A.; Marmet, C.; Fraering, A.L.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Dionisi, F.; Cavin, C.; et al. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: Small intestine and colon are key sites for coffee metabolism. Mol. Nutr. Food Res. 2010, 54, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of covalently bound ferulic acid from fiber in the human colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.M.; Souquet, J.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Gumienna, M.; Lasik, M.; Czarnecki, Z. Bioconversion of grape and chokeberry wine polyphenols during simulated gastrointestinal in vitro digestion. Int. J. Food Sci. Nutr. 2011, 62, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Molly, K.; Woestyne, M.V.; Verstraete, W. Development of a 5-step multichamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ekbatan, S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Jiménez-Girón, A.; Muñoz-González, I.; Esteban-Fernández, A.; Gil-Sánchez, I.; Dueñas, M.; Martín-Alvarez, P.J.; Pozo-Bayón, M.A.; Bartolomé, B.; Moreno-Arribas, M.V. Application of a new dynamic gastrointestinal simulator (SIMGI) to study the impact of red wine in colonic metabolism. Food Res. Int. 2015, 72, 149–159. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kubow, S.; Iskandar, M.M.; Melgar-Bermudez, E.; Sleno, L.; Sabally, K.; Azadi, B.; How, E.; Prakash, S.; Burgos, G.; Felde, T.Z. Effects of simulated human gastrointestinal digestion of two purple-fleshed potato cultivars on anthocyanin composition and cytotoxicity in colonic cancer and non-tumorigenic cells. Nutrients 2017, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Bresciani, L.; Dall’Asta, M.; Mena, P.; Del Rio, D.; Cid, C.; de Peña, M.-P. Bioaccessibility of (poly)phenolic compounds of raw and cooked cardoon (Cynara cardunculus L.) after simulated gastrointestinal digestion and fermentation by human colonic microbiota. J. Funct. Food 2017, 32, 195–207. [Google Scholar] [CrossRef]
- Ludwig, I.A.; de Peña, M.-P.; Cid, C.; Crozier, A. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; García-Conesa, M.T. Esterase activity to hydrolyze dietary antioxidant hydroxycinnamates is distributed along the intestine of mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Hitomi, Y.; Yoshioka, E. Intestinal absorption of p-coumaric and gallic acids in rats after oral administration. J. Agric. Food Chem. 2004, 52, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Peppercorn, M.A.; Goldman, P. Caffeic acid metabolism by gnotobiotic rats and their intestinal bacteria. PNAS 1972, 69, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Spencer, J.P.E.; Kuhnle, G.; Harn, U.; Rice-Evans, C.A. Novel biomarkers of the metabolism of caffeic acid derivatives in vivo. Free Radic. Biol. Med. 2001, 30, 1213–1222. [Google Scholar] [CrossRef]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; Van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, E.; Cueva, C.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. Development of human colonic microbiota in the computer-controlled dynamic simulator of the gastrointestinal tract SIMGI. LWT Food Sci. Technol. 2015, 61, 283–289. [Google Scholar] [CrossRef]
- Van Dorsten, F.A.; Peters, S.; Gross, G.; Gomez-Roldan, V.; Klinkenberg, M.; de Vos, R.C.; Vaughan, E.E.; van Duynhoven, J.P.; Possemiers, S.; van de Wiele, T.; et al. Gut microbial metabolism of polyphenols from black tea and red wine/grape juice is source-specific and colon-region dependent. J. Agric. Food Chem. 2012, 60, 11331–11342. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.A.; Leake, D.S.; Ames, J.M. In vitro antioxidant activity of coffee compounds and their metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar] [CrossRef] [PubMed]



| Theoretical Mass (m/z) 2 | Measured Mass | Mass Accuracy (ppm) | Retention Time (min) | Common Name | Systematic Name | AC | TC | DC | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T24 | T0 | T24 | T0 | T24 | ||||||
| 609.1461 | 609.1422 | 6.4 | 8.7 | Rutin | Quercetin-3-O-rutinoside | - | 9.62 | - | - | - | - |
| 353.0878 | 353.0863 | 4.3 | 7.5 | Chlorogenic acid | (1S,3R,4R,5R)-3-{[(2E)-3(3,4-Dihydroxyphenyl)prop-2enoyl]oxy}1,4,5trihydroxycyclohexanecarboxylic acid | - | 37.96 | - | - | - | - |
| 301.0354 | 301.0395 | 13.7 | 8 | Quercetin | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one | - | 1.05 | - | - | - | 0.15 |
| 193.0506 | 193.0499 | 3.8 | 8.5 | Ferulic acid | 3-(4-Hydroxy-3-methoxy-phenyl)prop-2-enoic acid | - | 2.03 | - | 0.16 | - | - |
| 191.0561 | 191.0544 | 2.3 | 1.7 | Quinic acid | (1S,3R,4S,5R)-1,3,4,5-Tetrahydroxycyclohexanecarboxylic acid | - | 642.68 | - | 28.74 | - | 1.39 |
| 181.0506 | 181.0505 | 0.7 | 7.7 | Dihydrocaffeic acid | 3- (3′,4′-Dihydroxyphenyl) propionic acid | - | 136.83 | 36.16 | 2.92 | 13.70 | 37.05 |
| 179.0325 | 179.339 | 6.1 | 8 | Caffeic acid | 3,4-Dihydroxycinnamic acid | - | 327.36 | - | - | - | 0.50 |
| 167.035 | 167.0346 | 2.2 | 6.6 | Vanillic acid | 4-Hydroxy-3-methoxybenzoic acid | - | 0.10 | - | 0.03 | - | 0.02 |
| 165.0557 | 165.0557 | 0.1 | 8.4 | 3-Hydroxylphenyl propionic acid | 3-(3′-Hydroxyphenyl)propionic acid | - | 36.69 | - | 28.59 | - | 7.56 |
| 163.0401 | 163.0401 | 0.2 | 8.4 | Coumaric acid | The isomer is not specified from our data | - | 12.34 | - | 1.05 | - | - |
| 153.0193 | 153.0204 | 6.9 | 7.2 | Protocatechuic acid | 3,4-Dihydroxybenzoic acid | - | 3.65 | - | 3.26 | - | 2.64 |
| 151.0401 | 151.0409 | 5.5 | 7.7 | 3-Hydroxyphenyl acetic acid | 3-Hydroxyphenylacetic acid 3 | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 149.0608 | 149.0604 | 2.7 | 9.4 | 3-Phenylpropionic acid | 3-Phenylpropionoic acid | - | 0.00 | 5.01 | 1.55 | 1.53 | 1.04 |
| 147.0452 | 147.0453 | 1 | 8.5 | Cinnamic acid | 3-Phenylprop-2-enoic acid | - | 0.74 | 0.46 | - | - | - |
| 137.0244 | 137.0245 | 0.6 | 7.2 | 3-Hydroxybenzoic acid | 3-Hydroxybenzoic acid | - | 9.02 | - | 8.88 | - | 3.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairallah, J.; Sadeghi Ekbatan, S.; Sabally, K.; Iskandar, M.M.; Hussain, R.; Nassar, A.; Sleno, L.; Rodes, L.; Prakash, S.; Donnelly, D.J.; et al. Microbial Biotransformation of a Polyphenol-Rich Potato Extract Affects Antioxidant Capacity in a Simulated Gastrointestinal Model. Antioxidants 2018, 7, 43. https://doi.org/10.3390/antiox7030043
Khairallah J, Sadeghi Ekbatan S, Sabally K, Iskandar MM, Hussain R, Nassar A, Sleno L, Rodes L, Prakash S, Donnelly DJ, et al. Microbial Biotransformation of a Polyphenol-Rich Potato Extract Affects Antioxidant Capacity in a Simulated Gastrointestinal Model. Antioxidants. 2018; 7(3):43. https://doi.org/10.3390/antiox7030043
Chicago/Turabian StyleKhairallah, Joelle, Shima Sadeghi Ekbatan, Kebba Sabally, Michèle M. Iskandar, Raza Hussain, Atef Nassar, Lekha Sleno, Laetitia Rodes, Satya Prakash, Danielle J. Donnelly, and et al. 2018. "Microbial Biotransformation of a Polyphenol-Rich Potato Extract Affects Antioxidant Capacity in a Simulated Gastrointestinal Model" Antioxidants 7, no. 3: 43. https://doi.org/10.3390/antiox7030043
APA StyleKhairallah, J., Sadeghi Ekbatan, S., Sabally, K., Iskandar, M. M., Hussain, R., Nassar, A., Sleno, L., Rodes, L., Prakash, S., Donnelly, D. J., & Kubow, S. (2018). Microbial Biotransformation of a Polyphenol-Rich Potato Extract Affects Antioxidant Capacity in a Simulated Gastrointestinal Model. Antioxidants, 7(3), 43. https://doi.org/10.3390/antiox7030043