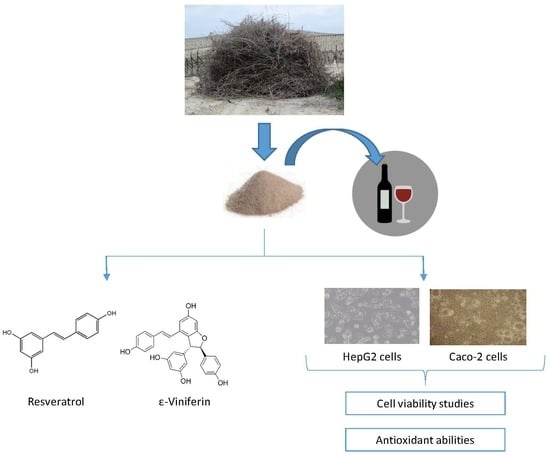
In Vitro Toxicity Assessment of Stilbene Extract for Its Potential Use as Antioxidant in the Wine Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Supplies, Chemicals and Model Systems
2.2. Stilbene-Enriched Extract
2.3. Test Solutions
2.4. Cytotoxicity Assays
2.5. Morphological Study under Light and Transmission Electron Microscope
2.6. Oxidative Stress and Antioxidant Ability Assays
2.7. Calculations and Statistical Analysis
3. Results
3.1. Presence of Stilbenes in the Extract
3.2. Cytotoxicity Studies
3.3. Light and Electron Microscopic Observation in HepG2 and Caco-2 Cells
3.4. Oxidative Stress Assays
3.5. Antioxidant Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Food. Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- EC, European Parliament and of the Council. Directive 2006/52/EC of the European Parliament and of the Council of 5 July 2006 amending directive 95/2/EC on food additives other than colours and sweeteners and Directive 94/35/EC on sweeteners for use in foodstuffs. Off. J. Eur. Union 2006, 204, 10–22. [Google Scholar]
- Cruz, S.; Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Gonzalo-Diago, A.; Moreno-Rojas, J.M.; Cantos-Villar, E. Grapevine-shoot stilbene extract as a preservative in white wine. Food Packag. Shelf Life 2018, 18, 164–172. [Google Scholar] [CrossRef]
- Santos, M.C.; Numes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape stem extracts Polyphenolic content and assessment of their in vitro antioxidant properties. LWT-Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Biais, B.; Krisa, S.; Cluzet, S.; Da Costa, G.; Waffo-Teguo, P.; Mérillon, J.M.; Richard, T. Antioxidant and Cytoprotective Activities of Grapevine Stilbenes. J. Agric. Food Chem. 2017, 65, 4952–4960. [Google Scholar] [CrossRef]
- Müller, C.; Ullmann, K.; Wilkens, A.; Winterhalter, P.; Toyokuni, S.; Steinberg, P. Potent Antioxidative Activity of Vineatrol®30 Grapevine-shoot Extract. Biosci. Biotechnol. Biochem. 2009, 73, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, M.J.; Raposo, R.; Cayuela, J.M.; Zafrilla, P.; Piñeiro, Z.; Moreno-Rojas, J.M.; Mulero, J.; Puertas, B.; Giron, F.; Guerrero, R.F.; et al. Valorization of grape stems. Ind. Crop. Prod. 2015, 63, 152–157. [Google Scholar] [CrossRef]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Gonzalo-Diago, A.; Guerrero, R.F.; Ortíz, V.; Cantos-Villar, E. Grapevine-shoot stilbene extract as a preservative in red wine. Food Chem. 2016, 197, 1102–1111. [Google Scholar] [CrossRef]
- Raposo, R.; Chinnici, F.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Carbú, M.; Guerrero, R.F.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Cantos-Villar, E. Sulfur free red wines through the use of grapevine shoots: Impact on the wine quality. Food Chem. 2018, 243, 453–460. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Guidance for submission for food additive evaluations. EFSA J. 2012, 10, 2760. [Google Scholar]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.M.; Cameán, A.M. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Radkar, V.; Hardej, D.; Lau-Cam, C.; Billack, B. Evaluation of resveratrol and piceatannol cytotoxicity in macrophages, T cells, and skin cells. Arhiv za Higijenu Rada i Toksikologiju 2007, 58, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Billard, C.; Izard, J.C.; Roman, V.; Kern, C.; Mathiot, C.; Mentz, F.; Kolb, J.P. Comparative Antiproliferative and Apoptotic Effects of resveratrol, ε-viniferin and vine-shots Derived Polyphenols (Vineatrols) on Chronic B Lymphocytic Leukemia Cells and Normal Human Lymphocytes. Leuk. Lymphoma 2002, 43, 1991–2002. [Google Scholar] [CrossRef]
- Marel, A.K.; Lizard, G.; Izard, J.C.; Latruffe, N.; Delmas, D. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar] [CrossRef]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, F.R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Wine, resveratrol and health: A review. Nat. Prod. Commun. 2009, 4, 635–658. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.M.; Monti, J.P. Protective effect of ε-viniferin on b-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg. Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef]
- Frombaum, M.; Le Clanche, S.; Bonnefont-Rousselot, D.; Borderie, D. Antioxidant effects of resveratrol and other stilbene derivatives on oxidative stress and NO bioavailability: Potential benefits to cardiovascular diseases. Biochimie 2012, 94, 269–276. [Google Scholar] [CrossRef]
- Ferguson, L.R. Role of plant polyphenols in genomic stability. Mutat. Res. 2001, 475, 89–111. [Google Scholar] [CrossRef]
- Ozkan, A.; Erdogan, A. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic components. Turk. J. Biol. 2011, 35, 735–742. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, A.; Moreno, F.J.; Cameán, A.M. Biochemical and pathological toxic effects induced by the cyanotoxin Cylindrospermopsin on the human cell line Caco-2. Water Res. 2012, 49, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; El Khawand, T.; Waffo-Teguo, P.; Decendit, A.; Richard, T.; Mérillon, J.M.; Roman, P. Stilbenes from grapevine root: A promising natural insecticide against Leptinotarsa decemlineata. J. Pest Sci. 2018, 91, 897–906. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Biais, B.; Richard, T.; Puertas, B.; Waffo-Teguo, P.; Merillon, J.M.; Cantos-Villar, E. Grapevine cane’s waste is a source of bioactive stilbenes. Ind. Crop. Prod. 2016, 94, 884–892. [Google Scholar] [CrossRef]
- Borenfreud, E.; Puerner, J.A. A simple quantitative procedure using monolayer culture for cytotoxicity assays. J. Tissue Cult. Methods 1984, 9, 7–9. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Lipsky, M.M.; Trump, B.F.; Hsu, I.C. Neutral red (NR) assay for cell viability and xenobiotic-induced cytotoxicity in primary cultures of human and rat hepatocytes. Cell Biol. Toxicol. 1990, 6, 219–234. [Google Scholar] [CrossRef]
- Baltrop, J.A.; Owen, T.C.; Cory, A.H. 5-((3-carboxyphenyl)-3-(4,5 dimethilthiazolyl)-3-(4- sulfophenyl) tetrazolium, inner salt (MTS) and related analogs of 2- (4,5- dimethylthiazolyl)- 2,5- diphenylterazolium bromide (MTT) reducing to purple water soluble formazan as cell viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Guzmán-Guillén, R.; Pichardo, S.; Moreno, F.J.; Vasconcelos, V.; Jos, A.; Cameán, A.M. Cytotoxic and morphological effects of microcystin-LR, cylindrospermopsin, and their combinations on the human hepatic cell line HepG2. Environ. Toxicol. 2019, 34, 240–251. [Google Scholar] [CrossRef]
- Wijeratne, S.S.K.; Cuppett, S.L.; Schlegel, V. Hydrogen Peroxide Induced Oxidative Stress Damage and Antioxidant Enzyme Response in Caco-2 Human Colon Cells. J. Agric. Food Chem. 2005, 53, 8768–8774. [Google Scholar] [CrossRef] [PubMed]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.H.; Wolfender, J.L.; Gindro, K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Gimazane, A.; Lizard, G.; Izard, J.C.; Solary, E.; Latruffe, N.; Delmas, D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5fluoro-uracil sensitivity in human derived colon cancer cells. Int. J. Cancer 2009, 124, 2780–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, C.S.; Ampomaah, W.; Mendonça, F.R.; Andrade, G.C.; Nazaré da Silva, A.M.; Goulart, M.O.; Alves dos Santos, R. Cytotoxicity and genotoxicity of stilbene derivatives in CHO-K1 and HepG2 cell lines. Genet. Mol. Biol. 2017, 40, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Empl, M.T.; Albers, M.; Wang, S.; Steinberg, P. The Resveratrol Tetramer r-Viniferin Induces a Cell Cycle Arrest Followed by Apoptosis in the Prostate Cancer Cell Line LNCaP. Phytother. Res. 2015, 29, 1640–1645. [Google Scholar] [CrossRef]
- Yilmazer, A. Cancer cell lines involving cancer stem cell populations respond to oxidative stress. Biotechnol. Rep. 2018, 17, 24–30. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. Chem. Med. Chem. 2017, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zheng, Z.; Liu, S.; Ren, G.; Gao, J.; Zhang, Y.; Li, G. Resveratrol promotes oxidative stress to drive DLC1 mediated cellular senescence in cancer cells. Exp. Cell Res. 2018, 370, 292–302. [Google Scholar] [CrossRef]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- De Bortoli, M.; Taverna, E.; Maffioli, E.; Casalini, P.; Crisafi, F.; Kumar, V.; Caccia, C.; Polli, D.; Tedeschi, G.; Bongarzone, I. Lipid accumulation in human breast cancer cells injured by iron depletors. J. Exp. Clin. Cancer Res. 2018, 37, 75. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, A.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. In Vitro 2015, 29, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In vitro and in vivo evaluation of the antioxidant and prooxidant activity of phenolic compounds obtained from grape (Vitis vinifera) pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
- Goutzourelas, N.; Stagos, D.; Spanidis, Y.; Liosi, M.; Apostolou, A.; Priftis, A.; Haroutounian, S.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 2015, 12, 5846–5856. [Google Scholar] [CrossRef] [PubMed]
- Goutzourelas, N.; Stagos, D.; Housmekeridou, A.; Karapouliou, C.; Kerasioti, E.; Aligiannis, N.; Skaltsounis, A.L.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. Int. J. Mol. Med. 2015, 36, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medrano-Padial, C.; Puerto, M.; Moreno, F.J.; Richard, T.; Cantos-Villar, E.; Pichardo, S. In Vitro Toxicity Assessment of Stilbene Extract for Its Potential Use as Antioxidant in the Wine Industry. Antioxidants 2019, 8, 467. https://doi.org/10.3390/antiox8100467
Medrano-Padial C, Puerto M, Moreno FJ, Richard T, Cantos-Villar E, Pichardo S. In Vitro Toxicity Assessment of Stilbene Extract for Its Potential Use as Antioxidant in the Wine Industry. Antioxidants. 2019; 8(10):467. https://doi.org/10.3390/antiox8100467
Chicago/Turabian StyleMedrano-Padial, Concepción, María Puerto, F. Javier Moreno, Tristan Richard, Emma Cantos-Villar, and Silvia Pichardo. 2019. "In Vitro Toxicity Assessment of Stilbene Extract for Its Potential Use as Antioxidant in the Wine Industry" Antioxidants 8, no. 10: 467. https://doi.org/10.3390/antiox8100467
APA StyleMedrano-Padial, C., Puerto, M., Moreno, F. J., Richard, T., Cantos-Villar, E., & Pichardo, S. (2019). In Vitro Toxicity Assessment of Stilbene Extract for Its Potential Use as Antioxidant in the Wine Industry. Antioxidants, 8(10), 467. https://doi.org/10.3390/antiox8100467