Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Buffer Preparation
2.3. Preparation of Ascorbic Acid Modified Halloysite (HNT/AH2)
2.4. Preparation of Ascorbic Acid Homogeneous Mixtures (M−x:AH2 + HNT)
2.5. Thermogravimetric Analysis (TGA)
2.6. Dynamic Light Scattering
2.7. UV-Vis Spectroscopy
2.8. Release of AH2 from HNT/AH2
2.9. Stability Studies of AH2
2.10. Determination of DPPH• Scavenging
2.11. Autoxidation Experiments
3. Results and Discussion
3.1. Preparation and Characterization of HNT/AH2
3.2. Release of Ascorbic Acid from HNT/AH2
3.3. Stability of Ascorbic Acid (AH2) in Solution
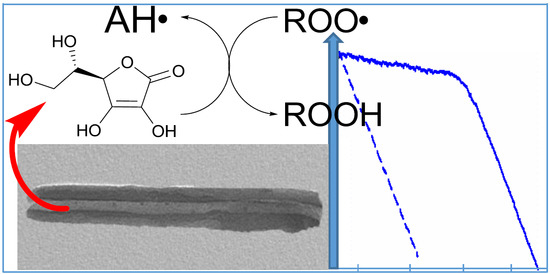
3.4. Radical Trapping and Antioxidant Activity of HNT/AH2
3.4.1. DPPH• Radical Trapping
3.4.2. Antioxidant Activity (ROO• Radical Trapping)
4. Conclusions
Supplementary Materialss
Author Contributions
Funding
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-1987-1748-5. [Google Scholar]
- Niki, E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R. Ascorbic Acid: Biochemistry and Biomedical Cell Biology, in Subcellular Biochemistry; Springer: New York, NY, USA, 1996; Volume 25, ISBN 978-1-4613-0325-1. [Google Scholar]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Vitamin C Fact Sheet for Consumers. Available online: https://ods.od.nih.gov/factsheets/VitaminC-Consumer/ (accessed on 1 July 2017).
- Hathcock, J.N.; Azzi, A.; Blumberg, J.; Bray, T.; Dickinson, A.; Frei, B.; Jialal, I.; Johnston, C.S.; Kelly, F.J.; Kraemer, K.; et al. Vitamins E and C are safe across a broad range of intakes. Am. J. Clin. Nutr. 2005, 81, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, S.A.; Baptista, R.; Della Gatta, P.A.; Yousif, A.; Russell, A.P.; Wadley, G.D. High-dose vitamin C supplementation increases skeletal muscle vitamin C concentration and SVCT2 transporter expression but does not alter redox status in healthy males. Free Radic. Biol. Med. 2014, 77, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Khan, A.; Khattak, M.M.A.K. Biological significance of ascorbic acid (Vitamin C) in human health—A Review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Young, J.I.; Zuchner, S.; Wang, G. Regulation of the Epigenome by Vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Valgimigli, L.; Bartolomei, D.; Amorati, R.; Haidasz, E.; Hanthorn, J.J.; Nara, S.J.; Brinkhorst, J.; Pratt, D.A. 3-Pyridinols and 5-pyrimidinols: Tailor-made for use in synergistic radical-trapping co-antioxidant systems. Beilstein J. Org. Chem. 2013, 9, 2781–2792. [Google Scholar] [CrossRef] [Green Version]
- Bowry, V.W.; Ingold, K.U. The unexpected role of vitamin E (α-tocopherol) in the peroxidation of human low-density lipoprotein. Acc. Chem. Res. 1999, 32, 27–34. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Degradation of ascorbic acid in aqueous aolution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Gallarate, M.; Carlotti, M.E.; Trotta, M.; Bovo, S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int. J. Pharm. 1999, 188, 233–241. [Google Scholar] [CrossRef]
- Sapeia, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl radical reactions in water solution: A gym for proton-coupled electron-transfer theories. Chem. Eur. J. 2016, 22, 7924–7934. [Google Scholar] [CrossRef] [PubMed]
- Haidasz, E.A.; Van Kessel, A.T.M.; Pratt, D.A. A Continuous visible light spectrophotometric approach to accurately determine the reactivity of radical-trapping antioxidants. J. Org. Chem. 2016, 81, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Liu, L.; Li, J.; Du, G.; Chen, J. Functions, applications and production of 2-O-D-glucopyranosyl-L-ascorbic acid. Appl. Microbiol. Biotechnol. 2012, 95, 313–320. [Google Scholar] [CrossRef]
- Špiclin, P.; Homar, M.; Zupančič-Valant, A.; Gašperlin, M. Sodium Ascorbyl phosphate in topical microemulsions. Int. J. Pharm. 2003, 256, 65–73. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G.F.; Valgimigli, L. Kinetic and thermodynamic aspects of the chain-breaking antioxidant activity of ascorbic acid derivatives in non-aqueous media. Org. Biomol. Chem. 2011, 9, 3792–3800. [Google Scholar] [CrossRef]
- Špiclin, P.; Gašperlin, M.; Kmetec, V. Stability of ascorbyl palmitate in topical microemulsions. Int. J. Pharm. 2001, 222, 271–279. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G.; Gianeti, M.D.; Camargo, F.B., Jr.; Gaspar, L.R. Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: Stability studies and in vivo efficacy. Eur. J. Pharm. Biopharm. 2012, 82, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Compton, R.G. Investigation of single-drug-encapsulating liposomes using the nano-impact method. Angew. Chem. Int. Ed. 2014, 53, 13928–13930. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Lee, S.-Y.; Han, Y.-S.; Park, K.-C.; Choy, J.-H. Efficient transdermal penetration and improved stability of L-ascorbic acid encapsulated in an inorganic nanocapsule. Bull. Korean Chem. Soc. 2003, 24, 499–503. [Google Scholar]
- Lvov, Y.M.; Shchukin, D.G.; Mohwald, H.; Price, R.R. Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Covalently modified halloysite clay nanotubes: synthesis, properties, biological and medical applications. J. Mater. Chem. B 2017, 5, 2867–2882. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Amorati, R.; Cavallaro, G.; Guernelli, S.; Lazzara, G.; Milioto, S.; Noto, R.; Poma, P.; Riela, S. Direct chemical grafted curcumin on halloysite nanotubes as dual-responsive prodrug for pharmacological applications. Colloid Surf. B 2016, 140, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhao, D.; Yao, P.; Wang, W.; Zhang, L.; Lvov, Y. Highly Aging-resistant elastomers doped with antioxidant-loaded clay nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8156–8165. [Google Scholar] [CrossRef]
- Massaro, M.; Piana, S.; Colletti, C.G.; Noto, R.; Riela, S.; Baiamonte, C.; Giordano, C.; Pizzolanti, G.; Cavallaro, G.; Milioto, S.; et al. Multicavity halloysite-amphiphilic cyclodextrin hybrids for co-delivery of natural drugs into thyroid cancer cells. J. Mater. Chem. B 2015, 3, 4074–4081. [Google Scholar] [CrossRef]
- Vergaro, V.; Lvov, Y.M.; Leporatti, S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol. Biosci. 2012, 12, 1265–1271. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite–trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef] [Green Version]
- Fakhrullina, G.I.; Akhatova, F.S.; Lvov, Y.M.; Fakhrullin, R.F. Toxicity of halloysite clay nanotubes in vivo: A caenorhabditis elegans study. Environ. Sci. Nano 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Valgimigli, L.; Bascheri, A.; Amorati, R. Antioxidant sctivity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Valgimigli, L. Measuring antioxidant activity in bioorganic samples by the differential oxygen uptake apparatus: recent advances. J. Chem. 2017, 2017, 6369358. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; d’Ischia, M. 5-S-Lipoylhydroxytyrosol, a multidefense antioxidant featuring a solvent-tunable peroxyl radical-scavenging 3-thio-1,2-dihydroxybenzene motif. J. Org. Chem. 2013, 78, 9857–9864. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Lynett, P.T.; Valgimigli, L.; Pratt, D.A. The reaction of sulfenic acids with peroxyl radicals: insights into the radical-trapping antioxidant activity of plant-derived thiosulfinates. Chem. Eur. J. 2012, 18, 6370–6379. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Lucarini, M.; Peduli, G.F.; Ingold, K.U. Does β-carotene really protect vitamin E from oxidation? J. Am. Chem. Soc. 1997, 119, 8095–8096. [Google Scholar] [CrossRef]
- Lucarini, M.; Peduli, G.F.; Valgimigli, L. Do peroxyl radicals obey the principle that kinetic solvent effects on H-atom abstraction are independent of the nature of the abstracting radical? J. Org. Chem. 1998, 63, 4497–4499. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L.; Amorati, R.; Minisci, F. Thermochemical and kinetic studies of a bisphenol antioxidant. J. Org. Chem. 2001, 66, 5456–5462. [Google Scholar] [CrossRef] [PubMed]
- Hanthorn, J.J.; Valgimigli, L.; Pratt, D.A. Incorporation of ring nitrogens into diphenylamine antioxidants: Striking a balance between reactivity and stability. J. Am. Chem. Soc. 2012, 134, 8306–8309. [Google Scholar] [CrossRef] [PubMed]
- Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Lanzi, M.; Saraga, F.; Sambri, L.; Franchini, M.C.; Giorgini, L. New nitrogen-rich heterocycles for organo-modified bentonites as flame retardant fillers in epoxy resin nanocomposites. Polym. Eng. Sci. 2017, 57, 621–630. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Cheng, H.; Zhou, L.; Ye, Y.; Chen, J. Intercalated polyaniline/halloysite nanocomposites by a solvent-free mechanochemical method. Nano 2016, 11, 1650112. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Palmisano, G.; Parisi, F. Halloysite nanotube with fluorinated lumen: Non-foaming nanocontainer for storage and controlled release of oxygen in aqueous media. J. Colloid Interfaces Sci. 2014, 417, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.D.; Scollary, G.R. Ascorbic acid: A review of its chemistry and reactivity in relation to a wine environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, E.; Tanaka, H.; Ohmoto, T.; Choami, M. Analysis of the transformation products of dehydro-L-ascorbic acid by ion-pairing high performance liquid chromatography. Anal. Biochem. 1993, 214, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Cabelli, D.E.; Blelskl, B.H.J. Kinetics and mechanism for the oxidation of ascorbic acid/ascorbate by HO2/O2– radicals. A pulse radiolysis and stopped-flow photolysis study. J. Phys. Chem. 1983, 87, 1809–1812. [Google Scholar] [CrossRef]
- Wilson, R.J.; Beezer, A.E.; Mitchell, J.C. A Kinetic study of the oxidation of l-ascorbic acid (vitamin C) in solution using an isothermal microcalorimeter. Thermochim. Acta 1995, 264, 27–40. [Google Scholar] [CrossRef]
- Buettner, G.R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: Ascorbate as a test for catalytic metals. J. Biochem. Bioph. Meth. 1988, 16, 27–40. [Google Scholar] [CrossRef]
- Robertson, G.L.; Samaniego, C.M.L. Effect of initial dissolved oxygen levels on the degradation of ascorbic acid and the browning of lemon juice during storage. J. Food Sci. 1986, 51, 184–187. [Google Scholar] [CrossRef]
- Viglianisi, C.; Di Pilla, V.; Menichetti, S.; Rotello, V.M.; Candiani, G.; Malloggi, C.; Amorati, R. Linking an α-tocopherol derivative to cobalt(0) nanomagnets: Magnetically responsive antioxidants with superior radical trapping activity and reduced cytotoxicity. Chem. Eur. J. 2014, 20, 6857–6860. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Amin, D.; Messersmith, P.B.; Anthony, J.E.; Prud’homme, R.K. Polymer directed self-assembly of pH-responsive antioxidant nanoparticles. Langmuir 2015, 31, 3612–3620. [Google Scholar] [CrossRef] [PubMed]







| Samples | Moisture Content (wt%) 1 | Weight Loss from 130 to 800 °C (wt%) 2 | Estimated AH2 Fraction (%) 3 |
|---|---|---|---|
| HNT | 2.60 ± 0.05 | 13.80 ± 0.05 | 0 |
| HNT/AH2 | 2.10 ± 0.05 | 18.40 ± 0.05 | 4.6 ± 0.1 |
| M-1.0:AH2 + HNT | 2.00 ± 0.05 | 15.00 ± 0.05 | 1.2 ± 0.1 |
| M-4.4:AH2 + HNT | 2.20 ± 0.05 | 18.20 ± 0.05 | 4.4 ± 0.1 |
| Sample | MeCN 1 | MeCN + 1% Water 1 | Buffer pH = 7.4 2 | |||
|---|---|---|---|---|---|---|
| kinh/M−1 s−1 | n | kinh/M−1 s−1 | n | kinh/M−1 s−1 | n | |
| HNT | No inhib | / | No inhib | / | No inhib | / |
| AH2 | (2.5 ± 0.5) × 104 | Figure 5A | (3.1 ± 0.5) × 104 | Figure 5A | (1.7 ± 0.2) × 106 | Figure 5B |
| AH2 + HNT | (1.5 ± 0.3) × 104 | Figure 5A | (3.5 ± 0.5) × 104 | Figure 5A | (1.5 ± 0.2) × 106 | Figure 5B |
| HNT/AH2 3 | (8 ± 1) × 103 | Figure 5A 4 | (5.1 ± 0.5) × 104 5 | Figure 5A 4 | (1.4 ± 0.3) × 106 | Figure 5B 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants 2019, 8, 30. https://doi.org/10.3390/antiox8020030
Baschieri A, Amorati R, Benelli T, Mazzocchetti L, D’Angelo E, Valgimigli L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants. 2019; 8(2):30. https://doi.org/10.3390/antiox8020030
Chicago/Turabian StyleBaschieri, Andrea, Riccardo Amorati, Tiziana Benelli, Laura Mazzocchetti, Emanuele D’Angelo, and Luca Valgimigli. 2019. "Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes" Antioxidants 8, no. 2: 30. https://doi.org/10.3390/antiox8020030
APA StyleBaschieri, A., Amorati, R., Benelli, T., Mazzocchetti, L., D’Angelo, E., & Valgimigli, L. (2019). Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants, 8(2), 30. https://doi.org/10.3390/antiox8020030