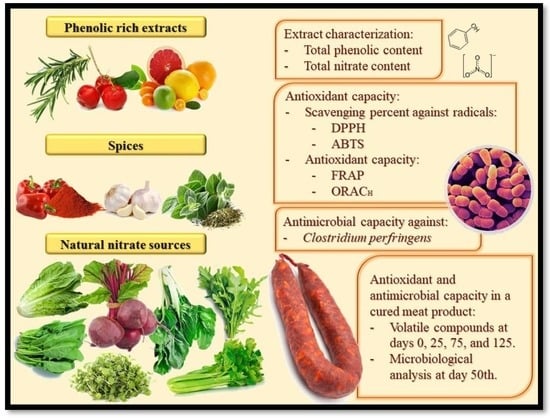
Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant, Spices, and Vegetable Extracts
2.1.1. Total Phenolic Content (TPC)
2.1.2. Total Nitrate Content (TNC)
2.1.3. Antioxidant Activity
2.1.4. Antimicrobial Activity
2.2. Cured Meat Product: Spanish Chorizo
2.2.1. Samples Preparation
2.2.2. Volatile Compounds by GC-MC
2.2.3. Microbiological Analysis
2.3. Statistical Analysis
3. Results
3.1. Characterization of Natural Extracts
3.1.1. Total Phenolic Content (TPC)
3.1.2. Total Nitrate Content (TNC)
3.1.3. Antioxidant Activity
3.1.4. Antimicrobial Activity
3.2. Volatile Compounds and Microbiological Spoilage in Spanish Chorizo
3.2.1. Volatile Compounds
3.2.2. Microbiological Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial activity and resistance: Influence factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Gammoh, S.; Al-Mahasneh, M.A.; Tranchant, C.C.; Rawshdeh, M. Chapter 15—Pharmaceutical nutraceutical and therapeutic properties of selected wild medicinal plants: Thyme, spearmint and rosemary. In Therapeutic, Probiotic and Unventional Food; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: New York, NY, USA, 2018; pp. 275–290. [Google Scholar]
- Andrade, M.A.; Ribeiro-Santos, R.; Costa-Bonito, M.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT Food Sci. Technol. 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Franco-Vega, A.; Reyes-Jurado, F.; Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Palou, E.; López-Malo, A. Chapter 89—Sweet orange (Citrus sinensis) oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: New York, NY, USA, 2016; pp. 783–790. [Google Scholar]
- Moura, C.F.H.; De Oliveira, L.S.; De Souza, K.O.; Da Franca, L.G.; Ribeiro, L.B.; De Souza, P.A.; De Miranda, M.R.A. Acerola—Malpiguia emarginata. In Exotic Fruits, Reference Guide; Academic Press: New York, NY, USA, 2018; pp. 7–14. [Google Scholar]
- Škrovánková, S.; Mlček, J.; Orsavová, J.; Juriková, T.; Dřímalová, P. Polyphenols content and antioxidant activity of paprika and pepper spices. Slovak J. Food Sci. 2017, 11, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Molnár, H.; Kónya, É.; Zalán, Z.; Bata-Vidács, I.; Tömösközi-Farkas, R.; Szèkács, A.; Adányi, N. Chemical characteristics of spice paprika of different origins. Food Control 2018, 83, 54–60. [Google Scholar] [CrossRef]
- Serrano, N.; Cetó, X.; Núñez, O.; Arago, M.; Gámez, A.; Ariño, C.; Díaz-Cruz, J.M. Characterization and classification of Spanish paprika (Capsicum annuum L.) by liquid chromatography coupled to electrochemical detection with screen-printed carbon-based nanomaterials electrodes. Talanta 2018, 189, 296–301. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, A.; Barros, L.; Ciric, A.; Sokovic, M.; Ferrerira, I.C.F.R. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Baranauskaite, J.; Kubiliene, A.; Marksa, M.; Petrikaite, V.; Vitkevičius, K.; Baranauskas, A.; Bernatoniene, J. The influence of different oregano species on the antioxidant activity determined using hplc postcolumn DPPH method and anticancer activity of carvacrol and rosmarinic acid. BioMed Res. Int. 2017, 2017, 1681392. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Azizi, F.; Ghasemi, A.; Hadaegh, F. Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meat and processed meats. J. Food Compos. Anal. 2016, 51, 93–105. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Milkowski, A.L. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 2012, 26, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Common Soil Sci. Plant Ann. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant avtivity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, S. Antioxidant activity applying and omproved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodil, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Ann. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Charlebois, A.; Jacques, M.; Archambault, M. Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials. Front. Microbiol. 2014, 5, 183. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Bedia, M.; Bañón, S. Relationship between flavor deterioration and the volatile compound profile of semi-ripened sausage. Meat Sci. 2013, 93, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Bhagwat, S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2; U.S. Department of Agriculture (USDA): Washington, DC, USA, 2010.
- EFSA. Nitrate in vegetables. Scientific opinion of the panel on contaminants in the food chain (Question No. EFSA-Q-2006-071). EFSA J. 2008, 689, 1–79. [Google Scholar]
- Brkić, D.; Bošnir, J.; Bevardi, M.; Gross-Bošković, A.; Miloš, S.; Lasić, D.; Krivohlavek, A.; Racz, A.; Mojsović-Ćuić, A.; Trstenjak, N.U. Nitrate in leafy green vegetables and estimated intake. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 31–41. [Google Scholar] [PubMed]
- Dobrinas, S.; Soceanu, A.; Popescu, V.; Stanciu, G. Nitrate determination in spices. Ovidius Univ. Ann. Chem. 2013, 24, 21–23. [Google Scholar]
- Hasan, S.M.; Hall, J.B. The physiological function of nitrate reduction in Clostridium perfringens. J. Gen. Microbiol. 1975, 87, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kerler, J.; Grosch, W. Character impact odorants of boiled chicken: Changes during refrigerated storage and reheating. Eur. Food Res. Technol. 1997, 205, 232–238. [Google Scholar] [CrossRef]
- Forss, D.A. Odor and flavor compounds from lipids. Prog. Chem. Fats Other Lipids 1973, 13, 177–258. [Google Scholar] [CrossRef]
- Meynier, A.; Novelli, E.; Chizzolini, R.; Zanardi, E.; Gandemer, G. Volatile compounds of commercial Milano salami. Meat Sci. 1999, 51, 175–183. [Google Scholar] [CrossRef]
- Tikk, K.; Haugen, J.E.; Andersen, H.; Aaslying, M. Monitoring of warmed-over flavor in pork using the electronic nose-Correlation to sensory attributes and secondary lipid oxidation products. Meat Sci. 2008, 80, 1254–1263. [Google Scholar] [CrossRef]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J.; Durcan, R. A comparison of solid-phase microextraction (SPME) fibres for measurement of hexanal and pentanal in cooked turkey. Food Chem. 2000, 68, 339–345. [Google Scholar] [CrossRef]
- Alfawaz, M.; Smith, J.S.; Jeon, I.J. Maillard reaction products as antioxidants in pre-cooked ground beef. Food Chem. 1994, 51, 311–318. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Lee, S.J.; Shin, K.M.; Park, E.J. The vegetation and flora of village groves in Paengseong-eup, Pyongtaek city, Gyonggi-Do, Korea. J. Korean Inst. Environ. Ecol. 2007, 21, 515–525. [Google Scholar]
- Ullrich, F.; Grosch, W. Identification of the most intense odor compounds formed during autoxidation of methyl linolenate at room temperature. J. Am. Oil Chem. Soc. 1988, 65, 1313–1317. [Google Scholar] [CrossRef]
- Ramarathnam, N.; Rubin, L.J.; Diosady, L.L. Studied on meat flavor. 3. A novel method for trapping volatile components from uncured and cured pork. J. Agric. Food Chem. 1993, 41, 933–938. [Google Scholar]
- Basmacioglu, H.; Tokusoglu, O.; Ergul, M. Effect of oregano and rosemary essential oils or alpha-tocopheryl acetate on performance and lipid oxidation of meat enriched with n-3 PUFA’s in broilers. S. Afr. J. Anim. Sci. 2004, 34, 197–210. [Google Scholar]
- Haak, L.; Raes, K.; Van Dyck, S.; De Smet, S. Effect of dietary rosemary and a-tocopheryl acetate on the oxidative stability of raw and cooked pork following oxidized linseed oil administration. Meat Sci. 2006, 78, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Dietary administration of ewe diets with a distillate from rosemary leaves (Rosmarinus officinalis L.): Influence on lamb meat quality. Meat Sci. 2010, 84, 23–29. [Google Scholar] [CrossRef]
- Gougoulias, N.; Wogiatzi, E.; Vagelas, I.; Giurgiulescu, L.; Gogou, I.; Ntalla, M.N.; Kalfountzos, D. Comparative study on polyphenols content, capsaicin and antioxidant activity of different hot peppers varieties (Capsicum annum, L.) under environmental conditions of Thessaly region, Greece. Carpathian J. Food Sci. Technol. 2017, 9, 109–116. [Google Scholar]
- Kruma, Z.; Andjelkovic, M.; Verhe, R.; Kreicbergs, V. Phenolic compounds in basil, oregano and thyme. In Proceedings of the 3rd Baltic Conference on Food Science and Technology, FOODBALT-2008, Jelgava, Latvia, 17–18 April 2008; pp. 99–103. [Google Scholar]
- Santos, R.D.; Shetty, K.; Cecchini, A.L.; da Silva-Miglioranza, L.H. Phenolic compounds and total antioxidant activity determination rosemary and oregano extracts and its use in cheese spread. Cienc. Agrárias Londrina 2012, 33, 655–666. [Google Scholar] [CrossRef]
- Zeb, A. Phenolic profile and antioxidant potential of wild watercress (Nasturtium officinale L.). SpringerPlus 2015, 4, 714. [Google Scholar] [CrossRef]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Storage stability of dietary nitrate and phenolic compounds in beetroot (Beta vulgaris) and arugula (Eruca sativa) juices. J. Food Sci. 2018, 83, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Flores, M.I.; Romero-González, R.; Martínez-Vidal, J.L.; Garrido-Frenich, A. Determination of phenolic compounds in artichoke, garlic and spinach by ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Food Anal. Methods 2014, 7, 2095–2106. [Google Scholar] [CrossRef]
- Pyo, Y.H.; Lee, T.C.; Logendra, L.; Rosen, R.T. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004, 85, 19–26. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Phenolic composition and antioxidant activities of 11 celery cultivars. J. Food Sci. 2010, 75, C9–C13. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, A.L.A.; Trugo, L.C. Phenolic compounds in acerola fruit (Malpighia punicifolia, L.). J. Braz. Chem. Soc. 2004, 15, 664–668. [Google Scholar] [CrossRef]
- Gardner, P.T.; White, T.A.; McPhail, D.B.; Duthie, G.G. The relative contribution of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000, 68, 471–474. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Cardoso, P.C.; Tomazini, A.P.B.; Stringheta, P.C.; Ribeiro, S.M.R.; Pinheiro-Sant’Ana, H.M. Vitamin C and carotenoids in organic and conventional fruits grown in Brazil. Food Chem. 2011, 126, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Ahn, J.; Lee, S.J.; Moon, B.; Ha, T.Y.; Kim, S. Phytochemicals and antioxidant activity of fruit and leaves of paprika (Capsicum Annuum L., var. special) cultived in Korea. J. Food Sci. 2011, 76, C193–C198. [Google Scholar] [CrossRef]
- Kim, J.S.; An, C.G.; Park, J.S.; Lim, Y.P.; Kim, S. Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colors, shapes and cultivation methods. Food Chem. 2016, 201, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, 716S–725S. [Google Scholar] [CrossRef] [PubMed]
- Saani, M.; Lawrence, R. Evaluation of pigments as antioxidant and antibacterial agents from Beta vulgaris linn. Int. J. Curr. Pharm. Res. 2017, 9, 37–41. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. J. Microbiol. 1983, 49, 209–224. [Google Scholar] [CrossRef]
- Dwidevi, B.K.; Snell, F.D. Meat flavor. Crit. Rev. Food Res. 1975, 5, 487–535. [Google Scholar]
- Ha, J.K.; Lindsay, R.C. Method for the quantitative analysis of volatile free and total branched-chain fatty acids in cheese and milk fat. J. Dairy Sci. 1990, 73, 1988–1999. [Google Scholar]
- Töth, L.; Potthast, K. Chemical aspects of the smoking of meat and meat products. Adv. Food Res. 1984, 29, 87–158. [Google Scholar]
- Nieto, G.; Estrada, M.; Jordán, M.J.; Garrido, M.D.; Bañón, S. Effects in ewe diet of rosemary by-product on lipid oxidation and the eating quality of cooked lamb under retail display conditions. Food Chem. 2011, 124, 1423–1429. [Google Scholar] [CrossRef]
- Nieto, G.; Bañon, S.; Garrido, M.D. Effectof supplementing ewes’diet with thyme (Thymus zygis ssp. Gracilis) leaves on the lipid oxidation of cooked lamb meat. Food Chem. 2011, 125, 1147–1152. [Google Scholar] [CrossRef]
- Johansson, G.; Berdagué, J.L.; Larsson, M.; Tran, N.; Borch, E. Lipolysis, proteolysis and formation of volatile components during ripening of a fermented sausage with Pediococcus pentosaceus and Staphylococcus xylosus as starter cultures. Meat Sci. 1994, 38, 203–218. [Google Scholar] [CrossRef]
- Frankel, E.N. Recent advances in lipid oxidation. J. Food Agric. 1991, 54, 495–511. [Google Scholar] [CrossRef]
- Berdagué, J.L.; Monteil, P.; Montel, M.C.; Talon, R. Effects of starter cultures on the formation of flavour compounds in dry sausage. Meat Sci. 1993, 35, 275–287. [Google Scholar] [CrossRef]
- Maga, J.A. The flavor chemistry of wood smoke. Food Rev. Int. 1987, 3, 139–183. [Google Scholar] [CrossRef]
- Croizet, F.; Denoyer, C.; Tran, N.; Berdagué, J. Les composes volatils du saucisson sec. Evolution au cours de la maturation. Viandes Prod. Carnes 1992, 13, 167–170. [Google Scholar]
- Berger, R.G.; Macku, C.; German, J.B.; Shibamoto, T. Isolation and identification of dry salami volatiles. J. Food Sci. 1990, 55, 1239–1242. [Google Scholar] [CrossRef]

| Samples | |||||||
|---|---|---|---|---|---|---|---|
| Ingredients | Control | CLAW | CSCe | CChB | RLAW | RSCe | RChB |
| Pork meat (g) | 875 | 875 | 875 | 875 | 875 | 875 | 875 |
| Pork fat (g) | 1350 | 1350 | 1350 | 1350 | 1350 | 1350 | 1350 |
| Water (mL) | 75 | 75 | 75 | 75 | 75 | 75 | 75 |
| Commercial mix® (g/kg) | 65 | ||||||
| Paprika (g/kg) | 30 | 30 | 30 | 30 | 30 | 30 | |
| Oregano (g/kg) | 3 | 3 | 3 | 3 | 3 | 3 | |
| Garlic (g/kg) | 3 | 3 | 3 | 3 | 3 | 3 | |
| Dextrose (g/kg) | 3 | 3 | 3 | 3 | 3 | 3 | |
| Salt (g/kg) | 5 | 5 | 5 | 5 | 5 | 5 | |
| Meat protein (g/kg) | 23 | 23 | 23 | 23 | 23 | 23 | |
| Ferment (mL) | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Natural extracts (ppm): | |||||||
| Ct | 200 | 200 | 200 | ||||
| R | 200 | 200 | 200 | ||||
| Ac | 100 | 100 | 100 | 100 | 100 | 100 | |
| LAW | 3000 + 1500 + 1500 | 3000 + 1500 + 1500 | |||||
| SCe | 3000 + 3000 | 3000 + 3000 | |||||
| ChB | 3000 + 3000 | 3000 + 3000 |
| Samples | Total Phenolic Content | Total Nitrate Content | ||
|---|---|---|---|---|
| mg GAE 100 g−1 | mg GAE 100 g−1 [23] | ppm NO3− | ppm NO3− [24] | |
| Ct | 1683.7 ± 8.6 c | Nd | Nd | Nd |
| Ac | 57.67 ± 1.5 i | Nd | Nd | Nd |
| R | 1913 ± 29 a | 4980 | Nd | Nd |
| Paprika | 1707 ± 20.1 b | 1643 | 21.8 ± 0.5 i | 108 |
| Garlic | 87.3 ± 2.5 hi | 92 | 50.2 ± 0.7 h | 69 |
| Oregano | 1439.7 ± 7.5 d | 3789 | 51.5 ± 0.3 h | Nd |
| B | 215.3 ± 9.6 ef | 55 * | 1384.1 ± 1.2 a | 1852 * |
| L | 145.3 ± 5.1 fg | 90 * | 736.4 ± 0.9 f | 1324 * |
| A | 296.3 ± 5.7 ef | 125 * | 1160.5 ± 1.0 c | 4677 * |
| S | 255 ± 6 ef | 205 * | 948.8 ± 0.8 d | 1066 * |
| Ch | 278 ± 37 ef | Nd | 1213.4 ± 1.5 b | 1690 * |
| Ce | 80 ± 1 hi | 42 * | 921.3 ± 1.1 e | 1103 * |
| W | 334.7 ± 4 e | Nd | 472.9 ± 0.8 g | 136 * |
| Samples | Chelating Activity Percent (%) | Antioxidant Activity (µM TE g−1 ± SD) | |||
|---|---|---|---|---|---|
| ABTS | DPPH | ORAC | FRAP | ORAC [23] | |
| Ct | 15.4 ± 0.2 h | 8.45 ± 0.3 k | 4828.5 ± 19.9 d | 6004.7 ± 29.6 c | Nd |
| Ac | 46.5 ± 0.3 c | 78.3 ± 0.5 b | 16,80.7 ± 19.3 g | 1925.7 ± 28.7 f | Nd |
| R | 70.2 ± 0.1 b | 76.7 ± 1.7 c | 19,909.0 ± 59.8 a | 17,790 ± 53.3 a | 112,200 ** |
| Paprika | 21.1 ± 1.6 f | 48.7 ± 0.2 ef | 5746.0 ± 21.7 c | 2491.3 ± 17.1 e | 13,750 |
| Garlic | 25.4 ± 0.8 e | 51.5 ± 0.3 d | 1919.3 ± 23.4 g | 1915.7 ± 52.5 f | 450 |
| Oregano | 15.6 ± 0.5 h | 41.3 ± 0.2 j | 11,436.7 ± 27.5 b | 9355.3 ± 46.4 b | 13,970 |
| B | 85.7 ± 1.1 a | 90.2 ± 0.6 a | 3509.0 ± 26.3 e | 3690 ± 58.8 d | 1946 * |
| L | 14.6 ± 1.1 i | 49.9 ± 0.1 e | 1723.3 ± 35.1 g | 1998 ± 18.9 f | 1321 * |
| A | 25.9 ± 3.1 e | 49.2 ± 1.2 e | 2881.3 ± 28.4 f | 2071 ± 16.3 ef | 1904 * |
| S | 20.1 ± 0.1 g | 43.6 ± 3.6 i | 1491.3 ± 22.1 gh | 1995.3 ± 9.6 f | 1513 * |
| Ch | 19.7 ± 0.0 g | 47.4 ± 0.6 g | 2150.7 ± 35.0 fg | 2216.7 ± 19.4 e | Nd |
| Ce | 12.0 ± 0.5 j | 48.7 ± 0.4 ef | 993.7 ± 18.5 i | 804.7 ± 33.6 g | 512 * |
| W | 33.4 ± 2.6 d | 46.5 ± 0.1 h | 1200.7 ± 15.0 h | 2510.3 ± 39.4 e | Nd |
| Volatile Compounds | Sample | Day 0 | Day 25 | Day 50 | Day 125 |
|---|---|---|---|---|---|
| propan-2-ol | Control | 0.45 ± 0.02 | 0.54 ± 0.02 | 1.02 ± 0.01 a | 1.75 ± 0.03 a |
| RLAW | 0.37 ± 0.01 | 0.46 ± 0.01 | 0.37 ± 0.02 b | 0.85 ± 0.04 c | |
| RSCe | 0.38 ± 0.03 | 0.44 ± 0.02 | 0.92 ± 0.01 b | 1.10 ± 0.05 b | |
| RChB | 0.58 ± 0.02 | 0.65 ± 0.01 | 0.66 ± 0.02 b | 1.27 ± 0.01 b | |
| CLAW | 0.65 ± 0.01 | 0.70 ± 0.01 | 0.89 ± 0.03 b | 1.20 ± 0.02 b | |
| CSCe | 0.61 ± 0.03 | 0.69 ± 0.03 | 0.59 ± 0.01 b | 1.33 ± 0.00 b | |
| CChB | 0.38 ± 0.02 | 0.50 ± 0.02 | 0.55 ± 0.01 b | 1.08 ± 0.01 b | |
| octen-2-ol | Control | 0.11 ± 0.01 | 0.10 ± 0.00 | 0.15 ± 0.01 | 0.10 ± 0.01 |
| RLAW | 0.10 ± 0.02 | 0.18 ± 0.02 | 0.15 ± 0.02 | 0.15 ± 0.02 | |
| RSCe | 0.14 ± 0.02 | 0.18 ± 0.01 | 0.12 ± 0.01 | 0.11 ± 0.01 | |
| RChB | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.16 ± 0.02 | 0.25 ± 0.02 | |
| CLAW | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.14 ± 0.03 | 0.19 ± 0.01 | |
| CSCe | 0.16 ± 0.00 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.02 | |
| CChB | 0.10 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.11 ± 0.01 | |
| Hexanal | Control | 0.11 ± 0.01 | 0.14 ± 0.02 | 0.21 ± 0.02 a | 0.44 ± 0.03 a |
| RLAW | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.08 ± 0.01 b | 0.18 ± 0.01 b | |
| RSCe | 0.10 ± 0.02 | 0.12 ± 0.01 | 0.12 ± 0.03 b | 0.18 ± 0.02 b | |
| RChB | 0.13 ± 0.01 | 0.16 ± 0.00 | 0.15 ± 0.02 b | 0.20 ± 0.01 b | |
| CLAW | 0.11 ± 0.02 | 0.14 ± 0.02 | 0.09 ± 0.00 b | 0.19 ± 0.02 b | |
| CSCe | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.18 ± 0.01 b | 0.21 ± 0.01 b | |
| CChB | 0.13 ± 0.03 | 0.12 ± 0.01 | 0.19 ± 0.01 b | 0.25 ± 0.02 b | |
| Nonanal | Control | 0.18 ± 0.01 | 0.39 ± 0.04 | 0.45 ± 0.02 a | 0.58 ± 0.01 a |
| RLAW | 0.17 ± 0.01 | 0.27 ± 0.03 | 0.32 ± 0.01 b | 0.41 ± 0.03 b | |
| RSCe | 0.22 ± 0.01 | 0.16 ± 0.01 | 0.27 ± 0.02 b | 0.27 ± 0.02 b | |
| RChB | 0.14 ± 0.01 | 0.18 ± 0.01 | 0.35 ± 0.01 b | 0.30 ± 0.02 b | |
| CLAW | 0.15 ± 0.02 | 0.18 ± 0.02 | 0.20 ± 0.03 b | 0.23 ± 0.01 b | |
| CSCe | 0.18 ± 0.03 | 0.15 ± 0.01 | 0.27 ± 0.02 b | 0.24 ± 0.02 b | |
| CChB | 0.17 ± 0.02 | 0.10 ± 0.01 | 0.19 ± 0.01 b | 0.25 ± 0.01 b |
| Samples | Analysis | ||
|---|---|---|---|
| TVC | TCC | Clostridium perfringens | |
| Control | 6.20 × 104 b | 2.77 × 102 | 10 a |
| RLAW | 5.12 × 105 a | 1.28 × 102 | Absence in 10 g b |
| RSCe | 4.25 × 105 a | 2.01 × 102 | Absence in 10 g b |
| RChB | 3.62 × 105 a | 1.10 × 102 | Absence in 10 g b |
| CLAW | 4.05 × 104 b | 1.56 × 102 | Absence in 10 g b |
| CSCe | 6.22 × 104 b | 1.79 × 102 | Absence in 10 g b |
| CChB | 5.98 × 104 b | 2.10 × 102 | Absence in 10 g b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants 2019, 8, 184. https://doi.org/10.3390/antiox8060184
Martínez L, Bastida P, Castillo J, Ros G, Nieto G. Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants. 2019; 8(6):184. https://doi.org/10.3390/antiox8060184
Chicago/Turabian StyleMartínez, Lorena, Pedro Bastida, Julian Castillo, Gaspar Ros, and Gema Nieto. 2019. "Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo" Antioxidants 8, no. 6: 184. https://doi.org/10.3390/antiox8060184
APA StyleMartínez, L., Bastida, P., Castillo, J., Ros, G., & Nieto, G. (2019). Green Alternatives to Synthetic Antioxidants, Antimicrobials, Nitrates, and Nitrites in Clean Label Spanish Chorizo. Antioxidants, 8(6), 184. https://doi.org/10.3390/antiox8060184