Eucalyptol Inhibits Amyloid-β-Induced Barrier Dysfunction in Glucose-Exposed Retinal Pigment Epithelial Cells and Diabetic Eyes
Abstract
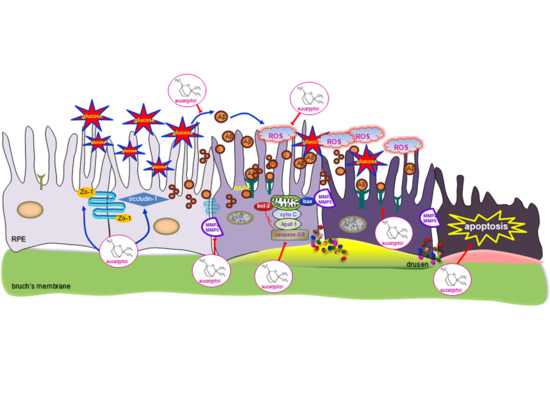
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RPE Cell Culture
2.3. In Vivo Animal Experiments
2.4. Western Blot Analysis
2.5. Hemotoxylin and Eosin (H&E) Staining
2.6. RT-PCR Analysis
2.7. ROS Generation
2.8. Immunocytochemical Staining
2.9. Hoechst 33258 Staining
2.10. Data Analysis
3. Results
3.1. Inhibition of Glucose-Induced Loss of Epithelial Junction Proteins by Eucalyptol
3.2. Blockade of Diabetic Disruption of Blood-Retinal Barrier by Eucalyptol
3.3. Anti-Apoptotic Role of Eucalyptol in Diabetic Rpe Cells and Retina
3.4. Suppression of Glucose-Induced Oxidative Stress by Eucalyptol
3.5. Inhibitory Effects of Eucalyptol on Glucose-Induced aβ Formation
3.6. Suppressive Effects of Eucalyptol on aβ-Induced Disruption of Tight Junctions
3.7. Blockade of aβ-Induced Apoptosis by Eucalyptol
3.8. Blockade of Aβ-RAGE Interaction by Eucalyptol
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- He, Y.; Zhang, Y.; Su, G. Recent advances in treatment of retinitis pigmentosa. Curr. Stem Cell Res. Ther. 2015, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Strong, S.; Liew, G.; Michaelides, M. Retinitis pigmentosa-associated cystoid macular oedema: Pathogenesis and avenues of intervention. Br. J. Ophthalmol. 2017, 101, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Humphries, P. The blood-retinal barrier: Tight junctions and barrier modulation. Adv. Exp. Med. Biol. 2012, 763, 70–84. [Google Scholar] [PubMed]
- Wang, W.; Kini, A.; Wang, Y.; Liu, T.; Chen, Y.; Vukmanic, E.; Emery, D.; Liu, Y.; Lu, X.; Jin, L.; et al. Metabolic deregulation of the blood-outer retinal barrier in retinitis pigmentosa. Cell Rep. 2019, 28, 1323–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.Z.; Song, Z.; Fu, S.; Zhu, M.; Le, Y.Z. RPE barrier breakdown in diabetic retinopathy: Seeing is believing. J. Ocul. Biol. Dis. Infor. 2011, 4, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Klaassen, I.; van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Xia, T.; Rizzolo, L.J. Effects of diabetic retinopathy on the barrier functions of the retinal pigment epithelium. Vis. Res. 2017, 139, 72–81. [Google Scholar] [CrossRef]
- Ponnalagu, M.; Subramani, M.; Jayadev, C.; Shetty, R.; Das, D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine 2017, 95, 126–135. [Google Scholar] [CrossRef]
- Rizzolo, L.J.; Peng, S.; Luo, Y.; Xiao, W. Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog. Retin. Eye Res. 2011, 30, 296–323. [Google Scholar] [CrossRef]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight junctions of the outer blood retina barrier. Int. J. Mol. Sci. 2019, 21, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liu, X. Roles of drug transporters in blood-retinal barrier. Adv. Exp. Med. Biol. 2019, 1141, 467–504. [Google Scholar] [PubMed]
- Giebel, S.J.; Menicucci, G.; McGuire, P.G.; Das, A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab. Investig. 2005, 85, 597–607. [Google Scholar] [CrossRef]
- Vinores, S.A.; Küchle, M.; Derevjanik, N.L.; Henderer, D.; Mahlow, J.; Green, W.R.; Campochiaro, P.A. Blood-retinal barrier breakdown in retinitis pigmentosa: Light and electron microscopic immunolocalization. Histol. Histopathol. 1995, 10, 913–923. [Google Scholar]
- D’Amico, A.G.; Maugeri, G.; Rasà, D.; Federico, C.; Saccone, S.; Lazzara, F.; Fidilio, A.; Drago, F.; Bucolo, C.; D’Agata, V. NAP modulates hyperglycemic-inflammatory event of diabetic retina by counteracting outer blood retinal barrier damage. J. Cell. Physiol. 2019, 234, 5230–5240. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, F.; Wang, W.; Zhang, X.D. Hydrogen sulfide attenuates high glucose-induced human retinal pigment epithelial cell inflammation by inhibiting Ros formation and NLRP3 inflammasome activation. Mediat. Inflamm. 2019, 2019, 8908960. [Google Scholar] [CrossRef] [Green Version]
- Harguindey, S.; Reshkin, S.J.; Orive, G.; Arranz, J.L.; Anitua, E. Growth and trophic factors, pH and the Na+/H+ exchanger in Alzheimer’s disease, other neurodegenerative diseases and cancer: New therapeutic possibilities and potential dangers. Curr. Alzheimer Res. 2007, 4, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Baglietto-Vargas, D.; Shi, J.; Yaeger, D.M.; Ager, R.; LaFerla, F.M. Diabetes and Alzheimer’s disease crosstalk. Neurosci. Biobehav. Rev. 2016, 64, 272–287. [Google Scholar] [CrossRef]
- Rad, S.K.; Arya, A.; Karimian, H.; Madhavan, P.; Rizwan, F.; Koshy, S.; Prabhu, G. Mechanism involved in insulin resistance via accumulation of β-amyloid and neurofibrillary tangles: Link between type 2 diabetes and Alzheimer’s disease. Drug Des. Dev. Ther. 2018, 12, 3999–4021. [Google Scholar]
- Takeda, S.; Sato, N.; Rakugi, H.; Morishita, R. Molecular mechanisms linking diabetes mellitus and Alzheimer disease: Beta-amyloid peptide, insulin signaling, and neuronal function. Mol. Biosyst. 2011, 7, 1822–1827. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Wijesekara, N.; Liyanapathirana, M.; Newsholme, P.; Ittner, L.; Fraser, P.; Verdile, G. The link between type 2 diabetes and neurodegeneration: Roles for amyloid-β, amylin, and tau proteins. J. Alzheimers Dis. 2017, 59, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dentchev, T.; Milam, A.H.; Lee, V.M.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003, 9, 184–190. [Google Scholar] [PubMed]
- Prasad, T.; Zhu, P.; Verma, A.; Chakrabarty, P.; Rosario, A.M.; Golde, T.E.; Li, Q. Amyloid β peptides overexpression in retinal pigment epithelial cells via AAV-mediated gene transfer mimics AMD-like pathology in mice. Sci. Rep. 2017, 7, 3222. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Tsujinaka, H.; Hirai, H.; Yamashita, M.; Ueda, T.; Ogata, N. Effects of concentration of amyloid β (Aβ) on viability of cultured retinal pigment epithelial cells. BMC Ophthalmol. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Sun, J.; Wang, F.; Luo, X.; Feng, J.; Gu, Q.; Liu, T.; Sun, X. MicroRNA Expression Patterns Involved in Amyloid Beta-Induced Retinal Degeneration. Invest. Ophthalmol. Vis. Sci. 2017, 58, 1726–1735. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhu, X.; Zhang, K.; Yao, Y.; Zhuang, M.; Tan, C.; Zhou, F.; Zhu, L. Puerarin inhibits amyloid β-induced NLRP3 Inflammasome Activation in retinal pigment epithelial cells via suppressing ROS-dependent oxidative and endoplasmic reticulum stresses. Exp. Cell. Res. 2017, 357, 335–340. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, S.M.; Salem, M.A.; Zayed, A.; et al. Insights into eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Seol, G.H.; Kim, K.Y. Eucalyptol and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 389–398. [Google Scholar]
- Kim, D.Y.; Kang, M.K.; Park, S.H.; Lee, E.J.; Kim, Y.H.; Oh, H.; Choi, Y.J.; Kang, Y.H. Eucalyptol ameliorates Snail1/β-catenin-dependent diabetic disjunction of renal tubular epithelial cells and tubulointerstitial fibrosis. Oncotarget 2017, 8, 106190–106205. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Kang, M.K.; Lee, E.J.; Kim, Y.H.; Oh, H.; Kang, Y.H. Eucalyptol inhibits advanced glycation end products-induced disruption of podocyte slit junctions by suppressing rage-erk-c-myc signaling pathway. Mol. Nutr. Food Res. 2018, 62, e1800302. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, G.; Siddiquei, M.M. Role of matrix metalloproteinase-2 and -9 in the development of diabetic retinopathy. J. Ocul. Biol. Dis. Infor. 2012, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zayit-Soudry, S.; Moroz, I.; Loewenstein, A. Retinal pigment epithelial detachment. Surv. Ophthalmol. 2007, 52, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: A novel therapeutic strategy for diabetic vascular complications. Expert. Opin. Investig. Drugs 2008, 17, 983–996. [Google Scholar] [CrossRef]
- Yan, S.D.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, F.; Du, Y.F.; Long, Y.; Reed, M.N.; Hu, M.; Suppiramaniam, V.; Hong, H.; Tang, S.S. Targeted inhibition of RAGE reduces amyloid-β influx across the blood-brain barrier and improves cognitive deficits in db/db mice. Neuropharmacology 2018, 131, 143–153. [Google Scholar] [CrossRef]
- Liu, J.; Jin, X.; Liu, K.J.; Liu, W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. 2012, 32, 3044–3057. [Google Scholar] [CrossRef]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of retinal cell death in diabetic retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef]
- Moreno, M.L.; Mérida, S.; Bosch-Morell, F.; Miranda, M.; Villar, V.M. Autophagy dysfunction and oxidative stress, two related mechanisms implicated in retinitis pigmentosa. Front. Physiol. 2018, 9, 1008. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Hong, S.H.; Kim, J.H.; Park, S.K.; Jeong, J.W.; Kim, G.Y.; Hyun, J.W.; Yun, J.S.; Kim, B.W.; et al. Protective effect of diphlorethohydroxycarmalol against oxidative stress-induced DNA damage and apoptosis in retinal pigment epithelial cells. Cutan. Ocul. Toxicol. 2019, 38, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid. Med. Cell Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Mann, S.N.; Mandal, N.A. Botanical compounds: Effects on major eye diseases. Evid. Based Complement. Altern. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emoto, Y.; Yoshizawa, K.; Uehara, N.; Kinoshita, Y.; Yuri, T.; Shikata, N.; Tsubura, A. Curcumin suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. In Vivo 2013, 27, 583–590. [Google Scholar] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Kopf, D.; Frolich, L. Risk of incident Alzheimer’s disease in diabetic patients: A systematic review of prospective trials. J. Alzheimers Dis. 2009, 16, 677–685. [Google Scholar] [CrossRef]
- Sundstrom, J.M.; Hernández, C.; Weber, S.R.; Zhao, Y.; Dunklebarger, M.; Tiberti, N.; Laremore, T.; Simó-Servat, O.; Garcia-Ramirez, M.; Barber, A.J.; et al. Proteomic analysis of early diabetic retinopathy reveals mediators of neurodegenerative brain diseases. Invest. Ophthalmol. Vis. Sci. 2018, 59, 2264–2274. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Vaibhav, K.; Javed, H.; Tabassum, R.; Ahmed, M.E.; Khan, M.M.; Khan, M.B.; Shrivastava, P.; Islam, F.; Siddiqui, M.S.; et al. 1,8-Cineole (eucalyptol) mitigates inflammation in amyloid beta toxicated pc12 cells: Relevance to Alzheimer’s disease. Neurochem. Res. 2014, 39, 344–352. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Zhang, S.; Song, W. High glucose promotes Aβ production by inhibiting APP degradation. PLoS ONE 2013, 8, e69824. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Song, W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Kang, M.-K.; Lee, E.-J.; Kim, Y.-H.; Oh, H.; Kim, S.-I.; Oh, S.Y.; Na, W.; Kang, Y.-H. Eucalyptol Inhibits Amyloid-β-Induced Barrier Dysfunction in Glucose-Exposed Retinal Pigment Epithelial Cells and Diabetic Eyes. Antioxidants 2020, 9, 1000. https://doi.org/10.3390/antiox9101000
Kim DY, Kang M-K, Lee E-J, Kim Y-H, Oh H, Kim S-I, Oh SY, Na W, Kang Y-H. Eucalyptol Inhibits Amyloid-β-Induced Barrier Dysfunction in Glucose-Exposed Retinal Pigment Epithelial Cells and Diabetic Eyes. Antioxidants. 2020; 9(10):1000. https://doi.org/10.3390/antiox9101000
Chicago/Turabian StyleKim, Dong Yeon, Min-Kyung Kang, Eun-Jung Lee, Yun-Ho Kim, Hyeongjoo Oh, Soo-Il Kim, Su Yeon Oh, Woojin Na, and Young-Hee Kang. 2020. "Eucalyptol Inhibits Amyloid-β-Induced Barrier Dysfunction in Glucose-Exposed Retinal Pigment Epithelial Cells and Diabetic Eyes" Antioxidants 9, no. 10: 1000. https://doi.org/10.3390/antiox9101000
APA StyleKim, D. Y., Kang, M. -K., Lee, E. -J., Kim, Y. -H., Oh, H., Kim, S. -I., Oh, S. Y., Na, W., & Kang, Y. -H. (2020). Eucalyptol Inhibits Amyloid-β-Induced Barrier Dysfunction in Glucose-Exposed Retinal Pigment Epithelial Cells and Diabetic Eyes. Antioxidants, 9(10), 1000. https://doi.org/10.3390/antiox9101000