The Association between Serum Bilirubin Levels and Colorectal Cancer Risk: Results from the Prospective Cooperative Health Research in the Region of Augsburg (KORA) Study in Germany
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population and Collection of Blood Samples and Data
2.2. Cancer Case Ascertainment and Selection
2.3. Laboratory Measurement of Circulating Bilirubin Concentrations
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Declarations
Ethics Approval and Consent to Participate
Abbreviations
| BMI | body mass index |
| CI | confidence intervals |
| CRC | colorectal cancer |
| EPIC | European Prospective Investigation into Cancer and nutrition |
| HPLC | high-performance liquid chromatography |
| ICD | International Classification of Diseases |
| KORA | Kooperative Gesundheitsforschung in der Region Augsburg (Cooperative Health Research in the Region of Augsburg) |
| NHANES | Third National Health and Nutrition Examination Survey |
| One-SD | one-standard-deviation |
| OR | odds ratios |
| ROS | reactive oxygen species |
| TA | thymine-adenine |
| UCB | unconjugated bilirubin |
| UGT1A1 | uridine diphosphate glucuronosyltransferase 1A1 |
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Wallner, M.; Molzer, C.; Gazzin, S.; Bulmer, A.C.; Tiribelli, C.; Vitek, L. Looking to the horizon: The role of bilirubin in the development and prevention of age-related chronic diseases. Clin. Sci. 2015, 129, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.M.; Sola, S.; Brito, M.A.; Brites, D.; Moura, J.J. Bilirubin directly disrupts membrane lipid polarity and fluidity, protein order, and redox status in rat mitochondria. J. Hepatol. 2002, 36, 335–341. [Google Scholar] [CrossRef]
- Hansen, T.W.; Mathiesen, S.B.; Walaas, S.I. Bilirubin has widespread inhibitory effects on protein phosphorylation. Pediatr. Res. 1996, 39, 1072–1077. [Google Scholar] [CrossRef] [Green Version]
- Fevery, J. Bilirubin in clinical practice: A review. Liver Int. Off. J. Int. Assoc. Stud Liver 2008, 28, 592–605. [Google Scholar] [CrossRef]
- Bulmer, A.C.; Ried, K.; Coombes, J.S.; Blanchfield, J.T.; Toth, I.; Wagner, K.H. The anti-mutagenic and antioxidant effects of bile pigments in the Ames Salmonella test. Mutat. Res. 2007, 629, 122–132. [Google Scholar] [CrossRef]
- Molzer, C.; Huber, H.; Diem, K.; Wallner, M.; Bulmer, A.C.; Wagner, K.H. Extracellular and intracellular anti-mutagenic effects of bile pigments in the Salmonella typhimurium reverse mutation assay. Toxicol. Int. J. Public Assoc. BIBRA 2013, 27, 433–437. [Google Scholar] [CrossRef]
- Molzer, C.; Huber, H.; Steyrer, A.; Ziesel, G.; Ertl, A.; Plavotic, A.; Wallner, M.; Bulmer, A.C.; Wagner, K.H. In vitro antioxidant capacity and antigenotoxic properties of protoporphyrin and structurally related tetrapyrroles. Free Radic. Res. 2012, 46, 1369–1377. [Google Scholar] [CrossRef]
- Molzer, C.; Huber, H.; Steyrer, A.; Ziesel, G.V.; Wallner, M.; Hong, H.T.; Blanchfield, J.T.; Bulmer, A.C.; Wagner, K.H. Bilirubin and related tetrapyrroles inhibit food-borne mutagenesis: A mechanism for antigenotoxic action against a model epoxide. J. Nat. Prod. 2013, 76, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Molzer, C.; Pfleger, B.; Putz, E.; Rossmann, A.; Schwarz, U.; Wallner, M.; Bulmer, A.C.; Wagner, K.H. In vitro DNA-damaging effects of intestinal and related tetrapyrroles in human cancer cells. Exp. Cell Res. 2013, 319, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Danoff, T.M.; Campbell, D.A.; McCarthy, L.C.; Lewis, K.F.; Repasch, M.H.; Saunders, A.M.; Spurr, N.K.; Purvis, I.J.; Roses, A.D.; Xu, C.F. A Gilbert’s syndrome UGT1A1 variant confers susceptibility to tranilast-induced hyperbilirubinemia. Pharm. J. 2004, 4, 49–53. [Google Scholar] [CrossRef] [Green Version]
- La Torre, A.; Targioni, G.; Rubaltelli, F.F. Beta-glucuronidase and hyperbilirubinemia in breast-Fed babies. Neonatology 1999, 75, 82–84. [Google Scholar] [CrossRef]
- Saxerholt, H.; Midtvedt, T. Intestinal deconjugation of bilirubin in germfree and conventional rats. Scand. J. Clin. Lab. Investig. 1986, 46, 341–344. [Google Scholar] [CrossRef]
- Gil, J.; Sasiadek, M.M. Gilbert syndrome: The UGT1A1*28 promoter polymorphism as a biomarker of multifactorial diseases and drug metabolism. Biomark. Med. 2012, 6, 223–230. [Google Scholar] [CrossRef]
- Tang, K.S.; Chiu, H.F.; Chen, H.H.; Eng, H.L.; Tsai, C.J.; Teng, H.C.; Huang, C.S. Link between colorectal cancer and polymorphisms in the uridine-diphosphoglucuronosyltransferase 1A7 and 1A1 genes. World J. Gastroenterol. 2005, 11, 3250–3254. [Google Scholar] [CrossRef]
- Jiraskova, A.; Novotny, J.; Novotny, L.; Vodicka, P.; Pardini, B.; Naccarati, A.; Schwertner, H.A.; Hubacek, J.A.; Puncocharova, L.; Smerhovsky, Z.; et al. Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. Int. J. Cancer 2012, 131, 1549–1555. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Liou, I.W.; Weiss, N.S. Serum bilirubin and colorectal cancer risk: A population-based cohort study. Aliment. Pharmacol. Ther. 2006, 23, 1637–1642. [Google Scholar] [CrossRef]
- Bajro, M.H.; Josifovski, T.; Panovski, M.; Jankulovski, N.; Nestorovska, A.K.; Matevska, N.; Petrusevska, N.; Dimovski, A.J. Promoter length polymorphism in UGT1A1 and the risk of sporadic colorectal cancer. Cancer Gen. 2012, 205, 163–167. [Google Scholar] [CrossRef]
- Girard, H.; Butler, L.M.; Villeneuve, L.; Millikan, R.C.; Sinha, R.; Sandler, R.S.; Guillemette, C. UGT1A1 and UGT1A9 functional variants, meat intake, and colon cancer, among Caucasians and African-Americans. Mutat. Res. 2008, 644, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, T.; Sookthai, D.; Graf, M.E.; Schubel, R.; Freisling, H.; Johnson, T.; Katzke, V.; Kaaks, R. Albumin, bilirubin, uric acid and cancer risk: Results from a prospective population-based study. Br. J. Cancer 2017, 117, 1572–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyed Khoei, N.; Jenab, M.; Murphy, N.; Banbury, B.L.; Carreras-Torres, R.; Viallon, V.; Kühn, T.; Bueno-de-Mesquita, B.; Aleksandrova, K.; Cross, A.J.; et al. Circulating bilirubin levels and risk of colorectal cancer: Serological and Mendelian randomization analyses. BMC Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Holle, R.; Happich, M.; Lowel, H.; Wichmann, H.E. KORA—A research platform for population based health research. Gesundh. Bundesverb. Arzte Off. Gesundh. Ger. 2005, 67 (Suppl. 1), S19–S25. [Google Scholar] [CrossRef] [Green Version]
- Wallner, M.; Bulmer, A.C.; Molzer, C.; Mullner, E.; Marculescu, R.; Doberer, D.; Wolzt, M.; Wagner, O.F.; Wagner, K.H. Haem catabolism: A novel modulator of inflammation in Gilbert’s syndrome. Eur. J. Clin. Investig. 2013, 43, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Molzer, C.; Wallner, M.; Kern, C.; Tosevska, A.; Zadnikar, R.; Doberer, D.; Marculescu, R.; Wagner, K.H. Characteristics of the heme catabolic pathway in mild unconjugated hyperbilirubinemia and their associations with inflammation and disease prevention. Sci. Rep. 2017, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.G. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef]
- Winkler, G.; Döring, A. Kurzmethoden zur Charakterisierung des Ernährungsmusters: Einsatz und Auswertung eines Food-Frequency-Fragebogens. Ernährungs Umschau 1995, 42, 289–291. [Google Scholar]
- Zucker, S.D.; Horn, P.S.; Sherman, K.E. Serum bilirubin levels in the U.S. population: Gender effect and inverse correlation with colorectal cancer. Hepatol. Baltim. Md. 2004, 40, 827–835. [Google Scholar] [CrossRef]
- Otero Regino, W.; Velasco, H.; Sandoval, H. The protective role of bilirubin in human beings. Rev. Colomb. Gastroenterol. 2009, 24, 293–301. [Google Scholar]
- Ollinger, R.; Kogler, P.; Troppmair, J.; Hermann, M.; Wurm, M.; Drasche, A.; Konigsrainer, I.; Amberger, A.; Weiss, H.; Ofner, D.; et al. Bilirubin inhibits tumor cell growth via activation of ERK. Cell Cycle Georgetown Tex. 2007, 6, 3078–3085. [Google Scholar] [CrossRef] [Green Version]
- Keshavan, P.; Schwemberger, S.J.; Smith, D.L.; Babcock, G.F.; Zucker, S.D. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer 2004, 112, 433–445. [Google Scholar] [CrossRef]
- Grant, D.J.; Bell, D.A. Bilirubin UDP-glucuronosyltransferase 1A1 gene polymorphisms: Susceptibility to oxidative damage and cancer? Mol. Carcinogen. 2000, 29, 198–204. [Google Scholar]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Seo, M.Y.; Lee, S.M. Protective effect of low dose of ascorbic acid on hepatobiliary function in hepatic ischemia/reperfusion in rats. J. Hepatol. 2002, 36, 72–77. [Google Scholar] [CrossRef]
- Wagner, K.H.; Shiels, R.G.; Lang, C.A.; Seyed Khoei, N.; Bulmer, A.C. Diagnostic criteria and contributors to Gilbert’s syndrome. Crit. Rev. Clin. Lab. Sci. 2018, 55, 129–139. [Google Scholar] [CrossRef]
- Roberts, M.C. Implementation Challenges for Risk-Stratified Screening in the Era of Precision Medicine. JAMA Oncol. 2018, 4, 1484–1485. [Google Scholar] [CrossRef]
| Parameters | Men | Women | ||||
|---|---|---|---|---|---|---|
| Case | Control | P | Case | Control | P | |
| N | 49 | 49 | 28 | 28 | ||
| Age at blood collection (years) | 59.2 (11.7) | 59.4 (11.8) | >0.9 | 58.5 (10.5) | 58.6 (10.6) | >0.9 |
| UCB (µmol/L) | 5.0 (3.5) | 5.7 (4.6) | 0.3 | 3.2 (2.2) | 3.9 (3.3) | 0.5 |
| Weight (kg) | 85.0 (12.9) | 82.2 (11.7) | 0.3 | 71.0 (13.2) | 72.0 (13.3) | 0.8 |
| Height (cm) | 171.7 (7.5) | 173.0 (6.1) | 0.4 | 159.7 (5.9) | 160.3 (7.4) | 0.8 |
| BMI (kg/m²) | 28.8 (3.7) | 27.5 (3.4) | 0.1 | 27.8 (4.8) | 28.0 (4.6) | 0.8 |
| Alcohol intake (g/day) | 34.3 (29.1) | 34.6 (28.5) | >0.9 | 11.1 (13.6) | 13.9 (16.9) | 0.7 |
| Smoking status (n, %) | 0.02 | 0.3 | ||||
| Current | 10 (26) | 6 (15) | 6 (22) | 2 (8) | ||
| Former | 22 (56) | 14 (36) | 4 (15) | 6 (23) | ||
| Never | 7 (18) | 19 (49) | 17 (63) | 18 (69) | ||
| Physical activity (n, %) † | 0.2 | 0.2 | ||||
| Active | 13 (34) | 19 (49) | 12 (44) | 7 (27) | ||
| Inactive | 25 (66) | 20 (51) | 15 (56) | 19 (73) | ||
| Menopause stage (n, %) | 0.6 | |||||
| Post-menopausal | 20 (83) | 19 (79) | ||||
| Pre-menopausal | 4 (17) | 4 (17) | ||||
| Use of HT (n, %) | >0.9 | |||||
| Yes | 3 (11) | 3 (11) | ||||
| No | 25 (89) | 25 (89) | ||||
| Healthy eating patterns (n, %) ‡ | 0.5 | 0.04 | ||||
| Healthy | 21 (64) | 23 (72) | 13 (62) | 18 (90) | ||
| Unhealthy | 12 (36) | 9 (28) | 8 (38) | 2 (10) | ||
| OR (95% CI) | |||||
|---|---|---|---|---|---|
| N Cases/ Controls | Crude | P | Adjusted | P | |
| Total | 77/77 | 0.83 (0.53–1.31) | 0.4 | 0.97 (0.50–1.86) | 0.9 |
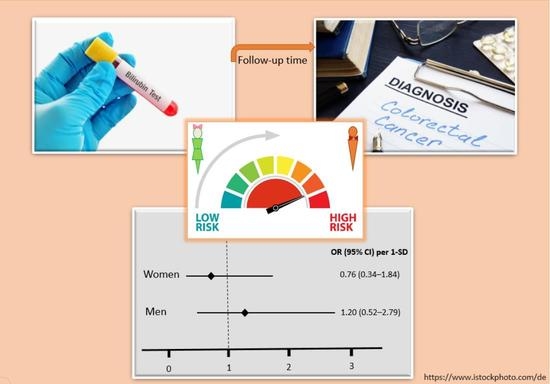
| Men | 49/49 | 0.81 (0.47–1.40) | 0.4 | 1.20 (0.52–2.79) | 0.7 |
| Women | 28/28 | 0.86 (0.48–1.56) | 0.6 | 0.76 (0.34–1.84) | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyed Khoei, N.; Anton, G.; Peters, A.; Freisling, H.; Wagner, K.-H. The Association between Serum Bilirubin Levels and Colorectal Cancer Risk: Results from the Prospective Cooperative Health Research in the Region of Augsburg (KORA) Study in Germany. Antioxidants 2020, 9, 908. https://doi.org/10.3390/antiox9100908
Seyed Khoei N, Anton G, Peters A, Freisling H, Wagner K-H. The Association between Serum Bilirubin Levels and Colorectal Cancer Risk: Results from the Prospective Cooperative Health Research in the Region of Augsburg (KORA) Study in Germany. Antioxidants. 2020; 9(10):908. https://doi.org/10.3390/antiox9100908
Chicago/Turabian StyleSeyed Khoei, Nazlisadat, Gabriele Anton, Annette Peters, Heinz Freisling, and Karl-Heinz Wagner. 2020. "The Association between Serum Bilirubin Levels and Colorectal Cancer Risk: Results from the Prospective Cooperative Health Research in the Region of Augsburg (KORA) Study in Germany" Antioxidants 9, no. 10: 908. https://doi.org/10.3390/antiox9100908
APA StyleSeyed Khoei, N., Anton, G., Peters, A., Freisling, H., & Wagner, K. -H. (2020). The Association between Serum Bilirubin Levels and Colorectal Cancer Risk: Results from the Prospective Cooperative Health Research in the Region of Augsburg (KORA) Study in Germany. Antioxidants, 9(10), 908. https://doi.org/10.3390/antiox9100908