The Industrial Organism Corynebacterium glutamicum Requires Mycothiol as Antioxidant to Resist Against Oxidative Stress in Bioreactor Cultivations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Culture Conditions
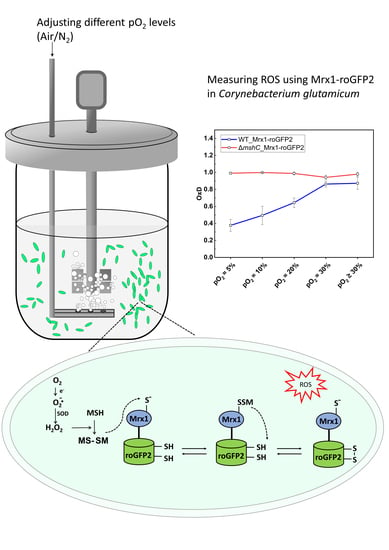
2.2. Fluorescence Measurements of Mrx1-roGFP2 Biosensor Oxidation In Vitro and In Vivo
2.3. Statistical Analysis
3. Results
3.1. The MSH-Deficient Mutant is Susceptible to Elevated Oxygen Concentrations
3.2. Oxidation of the Mrx1-roGFP2 Biosensor Allows Monitoring the Changes in the MSH Redox Potential (EMSH) in C. glutamicum
3.3. Mycothiol-Dependent Protection is Important when C. glutamicum is Exposed to Elevated Oxygen Concentrations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eggeling, L.; Bott, M. A giant market and a powerful metabolism: L-lysine provided by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2015, 99, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.A.E.; Wendisch, V.F. Engineering microbial cell factories: Metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products. Biotechnol. J. 2015, 10, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Wittmann, C. Industrial microorganisms: Corynebacterium glutamicum. Ind. Biotechnol. Microorg. 2017, 1, 183–220. [Google Scholar]
- Lange, J.; Münch, E.; Müller, J.; Busche, T.; Kalinowski, J.; Takors, R.; Blombach, B. Deciphering the adaptation of Corynebacterium glutamicum in transition from aerobiosis via microaerobiosis to anaerobiosis. Genes 2018, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Briki, A.; Kaboré, K.; Olmos, E.; Bosselaar, S.; Blanchard, F.; Fick, M.; Guedon, E.; Fournier, F.; Delaunay, S. Corynebacterium glutamicum, a natural overproducer of succinic acid? Eng. Life Sci. 2020, 20, 205–215. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.A. How obligatory is anaerobiosis? Mol. Microbiol. 2008, 68, 801–804. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Korshunov, S.; Imlay, J.A. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J. Bacteriol. 2006, 188, 6326–6334. [Google Scholar] [CrossRef] [Green Version]
- Messner, K.R.; Imlay, J.A. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 1999, 274, 10119–10128. [Google Scholar] [CrossRef] [Green Version]
- Antelmann, H.; Helmann, J.D. Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 2011, 14, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, J.; Vogt, S.L.; Reynolds, L.A.; Peña-Díaz, J.; Tupin, A.; Aussel, L.; Finlay, B.B. Exploring the redox balance inside Gram-negative bacteria with redox-sensitive GFP. Free Radic. Biol. Med. 2016, 91, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Seaver, L.C.; Imlay, J.A.; Loewen, P. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 2001, 183, 7173–7181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, M.; Xu, Y.; Wang, T.; Long, M.; Ding, W.; Chen, C.; Guan, X.; Liu, Y.; Wang, Y.; Shen, X.; et al. Functional characterization of a mycothiol peroxidase in Corynebacterium glutamicum that uses both mycoredoxin and thioredoxin reducing systems in the response to oxidative stress. Biochem. J. 2015, 469, 45–57. [Google Scholar] [CrossRef]
- Pedre, B.; Van Molle, I.; Villadangos, A.F.; Wahni, K.; Vertommen, D.; Turell, L.; Erdogan, H.; Mateos, L.M.; Messens, J. The Corynebacterium glutamicum mycothiol peroxidase is a reactive oxygen species-scavenging enzyme that shows promiscuity in thiol redox control. Mol. Microbiol. 2015, 96, 1176–1191. [Google Scholar] [CrossRef]
- Van Laer, K.; Hamilton, C.J.; Messens, J. Low-molecular-weight thiols in thiol-disulfide exchange. Antioxid. Redox Signal. 2013, 18, 1642–1653. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Karpukhina, O.V.; Kamzolova, S.V.; Samoilenko, V.A.; Inozemtsev, A.N. Investigation of the effect of biologically active threo-Ds-isocitric acid on oxidative stress in Paramecium caudatum. Prep. Biochem. Biotechnol. 2018, 48, 1–5. [Google Scholar] [CrossRef]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef]
- Reyes, A.M.; Pedre, B.; De Armas, M.I.; Tossounian, M.A.; Radi, R.; Messens, J.; Trujillo, M. Chemistry and redox biology of mycothiol. Antioxid. Redox Signal. 2018, 28, 487–504. [Google Scholar] [CrossRef]
- Tung, Q.N.; Linzner, N.; Van Loi, V.; Antelmann, H. Application of genetically encoded redox biosensors to measure dynamic changes in the glutathione, bacillithiol and mycothiol redox potentials in pathogenic bacteria. Free Radic. Biol. Med. 2018, 128, 84–96. [Google Scholar] [CrossRef]
- Jothivasan, V.K.; Hamilton, C.J. Mycothiol: Synthesis, biosynthesis and biological functions of the major low molecular weight thiol in actinomycetes. Nat. Prod. Rep. 2008, 25, 1091–1117. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.L.; Buchmeier, N.; Fahey, R.C. Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol. Mol. Biol. Rev. 2008, 72, 471–494. [Google Scholar] [CrossRef] [Green Version]
- Tossounian, M.A.; Pedre, B.; Wahni, K.; Erdogan, H.; Vertommen, D.; Van Molle, I.; Messens, J. Corynebacterium diphtheriae methionine sulfoxide reductase a exploits a unique mycothiol redox relay mechanism. J. Biol. Chem. 2015, 290, 11365–11375. [Google Scholar] [CrossRef] [Green Version]
- Si, M.; Zhao, C.; Zhang, B.; Wei, D.; Chen, K.; Yang, X.; Xiao, H.; Shen, X. Overexpression of mycothiol disulfide reductase enhances Corynebacterium glutamicum robustness by modulating cellular redox homeostasis and antioxidant proteins under oxidative stress. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Van Loi, V.; Rossius, M.; Antelmann, H. Redox regulation by reversible protein S-thiolation in bacteria. Front. Microbiol. 2015, 6, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hillion, M.; Antelmann, H. Thiol-based redox switches in prokaryotes. Biol. Chem. 2015, 396, 415–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, B.K.; Busche, T.; Van Laer, K.; Bäsell, K.; Becher, D.; Clermont, L.; Seibold, G.M.; Persicke, M.; Kalinowski, J.; Messens, J.; et al. Protein S-Mycothiolation functions as redox-switch and thiol protection mechanism in Corynebacterium glutamicum under hypochlorite stress. Antioxid. Redox Signal. 2014, 20, 589–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, X.; Yin, Y.; Lin, J.; Chen, C.; Pan, J.; Si, M.; Shen, X. Mycothiol protects Corynebacterium glutamicum against acid stress via maintaining intracellular pH homeostasis, scavenging ROS, and S-mycothiolating MetE. J. Gen. Appl. Microbiol. 2016, 62, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Tung, Q.N.; Van Loi, V.; Busche, T.; Nerlich, A.; Mieth, M.; Milse, J.; Kalinowski, J.; Hocke, A.C.; Antelmann, H. Stable integration of the Mrx1-roGFP2 biosensor to monitor dynamic changes of the mycothiol redox potential in Corynebacterium glutamicum. Redox Biol. 2019, 20. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Turrens, J.F.; Freeman, B.A.; Crapo, J.D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Biochem. Biophys. 1982, 217, 411–421. [Google Scholar] [CrossRef]
- Freeman, B.A.; Topolosky, M.K.; Crapo, J.D. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch. Biochem. Biophys. 1982, 216, 477–484. [Google Scholar] [CrossRef]
- Haugaard, N. Cellular mechanisms of oxygen toxicity. Physiol. Rev. 1968, 48, 311–373. [Google Scholar] [CrossRef] [PubMed]
- Boehme, D.E.; Vincent, K.; Brown, O.R. Oxygen and toxicity inhibition of amino acid biosynthesis. Nature 1976, 262, 418–420. [Google Scholar] [CrossRef]
- Gregory, E.M.; Fridovich, I. Oxygen toxicity and the superoxide dismutase. J. Bacteriol. 1973, 114, 1193–1197. [Google Scholar] [CrossRef] [Green Version]
- Käß, F.; Hariskos, I.; Michel, A.; Brandt, H.J.; Spann, R.; Junne, S.; Wiechert, W.; Neubauer, P.; Oldiges, M. Assessment of robustness against dissolved oxygen/substrate oscillations for Corynebacterium glutamicum DM1933 in two-compartment bioreactor. Bioprocess Biosyst. Eng. 2014, 37, 1151–1162. [Google Scholar] [CrossRef]
- Abe, S.; Takayama, K.I.; Kinoshita, S. Taxonomical studies on glutamic acid-producing bacteria. J. Gen. Appl. Microbiol. 1967, 13, 279–301. [Google Scholar] [CrossRef]
- Clermont, L.; Macha, A.; Müller, L.M.; Derya, S.M.; von Zaluskowski, P.; Eck, A.; Eikmanns, B.J.; Seibold, G.M. The α-glucan phosphorylase MalP of Corynebacterium glutamicum is subject to transcriptional regulation and competitive inhibition by ADP-glucose. J. Bacteriol. 2015, 197, 1394–1407. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.; Sobotta, M.C.; Dick, T.P. Measuring EGSH and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 2011, 51, 1943–1951. [Google Scholar] [CrossRef]
- Van Loi, V.; Harms, M.; Müller, M.; Huyen, N.T.T.; Hamilton, C.J.; Hochgräfe, F.; Pané-Farré, J.; Antelmann, H. Real-Time imaging of the bacillithiol redox potential in the human pathogen Staphylococcus aureus using a genetically encoded bacilliredoxin-fused redox biosensor. Antioxid. Redox Signal. 2017, 26, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004, 279, 22284–22293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, F.S.; Blombach, B.; Eikmanns, B.J. Metabolic engineering of Corynebacterium glutamicum for 2-Ketoisovalerate production. Appl. Environ. Microbiol. 2010, 76, 8053–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roenneke, B.; Rosenfeldt, N.; Derya, S.M.; Novak, J.F.; Marin, K.; Krämer, R.; Seibold, G.M. Production of the compatible solute α-d-glucosylglycerol by metabolically engineered Corynebacterium glutamicum. Microb. Cell Factories 2018, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Zieringer, J.; Haas, T.; Nieß, A.; Blombach, B.; Takors, R. Physiological response of Corynebacterium glutamicum to increasingly nutrient-rich growth conditions. Front. Microbiol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Zha, J.; Cheng, H.; Ibrahim, M.H.A.; Yang, F.; Dalton, H.; Cao, R.; Zhu, Y.; Fang, J.; Chi, K.; et al. Engineering Corynebacterium glutamicum for the de novo biosynthesis of tailored poly-γ-glutamic acid. Metab. Eng. 2019, 56, 39–49. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Dick, T.P.; Meyer, A.J.; Morgan, B. Dissecting redox biology using fluorescent protein sensors. Antioxid. Redox Signal. 2016, 24, 680–712. [Google Scholar] [CrossRef]
- Van Laer, K.; Buts, L.; Foloppe, N.; Vertommen, D.; Van Belle, K.; Wahni, K.; Roos, G.; Nilsson, L.; Mateos, L.M.; Rawat, M.; et al. Mycoredoxin-1 is one of the missing links in the oxidative stress defence mechanism of Mycobacteria. Mol. Microbiol. 2012, 86, 787–804. [Google Scholar] [CrossRef]
- Liu, Y.B.; Long, M.X.; Yin, Y.J.; Si, M.R.; Zhang, L.; Lu, Z.Q.; Wang, Y.; Shen, X.H. Physiological roles of mycothiol in detoxification and tolerance to multiple poisonous chemicals in Corynebacterium glutamicum. Arch. Microbiol. 2013, 195, 419–429. [Google Scholar] [CrossRef]
- El Shafey, H.M.; Ghanem, S.; Merkamm, M.; Guyonvarch, A. Corynebacterium glutamicum superoxide dismutase is a manganese-strict non-cambialistic enzyme in vitro. Microbiol. Res. 2008, 163, 80–86. [Google Scholar] [CrossRef]
- Si, M.; Feng, Y.; Chen, K.; Kang, Y.; Chen, C.; Wang, Y.; Shen, X. Functional comparison of methionine sulphoxide reductase A and B in Corynebacterium glutamicum. J. Gen. Appl. Microbiol. 2017, 63, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Si, M.; Zhang, L.; Chaudhry, M.T.; Ding, W.; Xu, Y.; Chen, C.; Akbar, A.; Shen, X.; Liu, S.J. Corynebacterium glutamicum methionine sulfoxide reductase a uses both mycoredoxin and thioredoxin for regeneration and oxidative stress resistance. Appl. Environ. Microbiol. 2015, 81, 2781–2796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baez, A.; Shiloach, J. Escherichia coli avoids high dissolved oxygen stress by activation of SoxRS and manganese-superoxide dismutase. Microb. Cell Factories 2013, 12, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, M.; Wang, T.; Pan, J.; Lin, J.; Chen, C.; Wei, Y.; Lu, Z.; Wei, G.; Shen, X. Graded response of the multifunctional 2-cysteine peroxiredoxin, CgPrx, to increasing levels of hydrogen peroxide in Corynebacterium glutamicum. Antioxid. Redox Signal. 2017, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]



| Shake Flask | EMSH (mV) | |
|---|---|---|
| WT_Mrx1-roGFP2 (a) | ΔmshC_Mrx1-roGFP2 (b) | |
| Initial value | −274 ± 2 | −248 ± 2 |
| End of exponential growth phase | −280 ± 2 | −255 ± 7 |
| Bioreactor pO2 level (%) | EMSH (mV) | |
|---|---|---|
| WT_Mrx1-roGFP2 (a) | ΔmshC_Mrx1-roGFP2 (b) | |
| 5 | −287 ± 4 | −204 ± 2 |
| 20 | −280 ± 6 | −191 ± 2 |
| 25 | −272 ± 3 | −208 ± 22 |
| 30% | −256 ± 4 | −242 ± 7 |
| ≥30% | −246 ± 28 | −218 ± 23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartmann, F.S.F.; Clermont, L.; Tung, Q.N.; Antelmann, H.; Seibold, G.M. The Industrial Organism Corynebacterium glutamicum Requires Mycothiol as Antioxidant to Resist Against Oxidative Stress in Bioreactor Cultivations. Antioxidants 2020, 9, 969. https://doi.org/10.3390/antiox9100969
Hartmann FSF, Clermont L, Tung QN, Antelmann H, Seibold GM. The Industrial Organism Corynebacterium glutamicum Requires Mycothiol as Antioxidant to Resist Against Oxidative Stress in Bioreactor Cultivations. Antioxidants. 2020; 9(10):969. https://doi.org/10.3390/antiox9100969
Chicago/Turabian StyleHartmann, Fabian Stefan Franz, Lina Clermont, Quach Ngoc Tung, Haike Antelmann, and Gerd Michael Seibold. 2020. "The Industrial Organism Corynebacterium glutamicum Requires Mycothiol as Antioxidant to Resist Against Oxidative Stress in Bioreactor Cultivations" Antioxidants 9, no. 10: 969. https://doi.org/10.3390/antiox9100969
APA StyleHartmann, F. S. F., Clermont, L., Tung, Q. N., Antelmann, H., & Seibold, G. M. (2020). The Industrial Organism Corynebacterium glutamicum Requires Mycothiol as Antioxidant to Resist Against Oxidative Stress in Bioreactor Cultivations. Antioxidants, 9(10), 969. https://doi.org/10.3390/antiox9100969