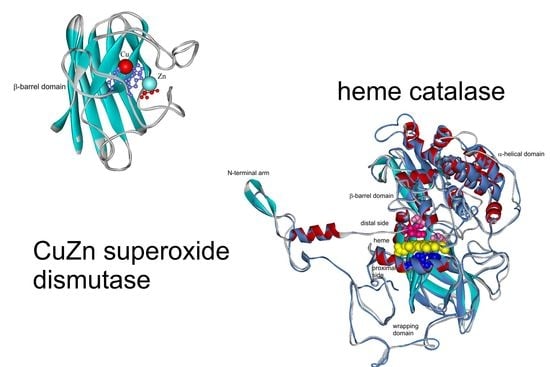
Parallel Molecular Evolution of Catalases and Superoxide Dismutases—Focus on Thermophilic Fungal Genomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Material and Isolation of Genomic DNA
2.2. Whole Genome Sequencing and ORF Prediction
2.3. Detection of Native Expression of SOD and CAT Gene Paralogs from mRNA Libraries
2.4. Multiple Sequence Alignment and Phylogenetic Reconstruction
2.5. Modeling of Structures for Antioxidant Enzymes
3. Results and Discussion
3.1. Genomic Analyses and Detection of Specific mRNAs for Fungal Antioxidant-Coding Genes
3.2. Superoxide Dismutases (SOD, EC 1.15.1.1)
3.2.1. Copper-Zinc Superoxide Dismutases
3.2.2. Iron-Manganese Superoxide Dismutases
3.3. Catalases (CAT, EC 1.11.1.6)
3.4. Outlook and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peschek, G.A.; Obinger, C.; Renger, G. Bioenergetic Processes of Cyanobacteria—From Evolutionary Singularity to Ecological Diversity; Springer: New York, NY, USA, 2011; ISBN 978-94-007-0352-0. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.-P.; Fleury, M.J.J. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef]
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen Peroxide and Redox Regulation of Developments. Antioxidants 2018, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Dai, D.-F.; Chiao, Y.-A.; Martin, G.M.; Marcinek, D.J.; Basisty, N.; Quarles, E.K.; Rabinovitch, P.S. Mitochondrial-targeted catalase: Extended longevity and the roles in various disease models. Prog. Mol. Biol. Transl. 2017, 146, 203–242. [Google Scholar] [CrossRef]
- Ekoue, D.N.; He, C.; Diamond, A.M.; Bonini, M. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim. Biophys. Acta 2017, 1858, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Hansberg, W.; Salas-Lizana, R.; Dominguez, L. Fungal catalases: Function, phylogenetic origin and structure. Arch. Biochem. Biophys. 2012, 525, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ling, Z.; Khan, A.; Virk, A.K.; Kulshrestha, S.; Li, X. Improvements of thermophilic enzymes: From genetic modifications to applications. Bioresour. Technol. 2019, 279, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Thanh, V.N.; Thuy, N.T.; Huong, H.T.T.; Hien, D.D.; Hang, D.T.M.; Anh, D.T.K.; Hüttner, S.; Larsbrink, J.; Olsson, L. Surveying of acid-tolerant thermophilic lignocellulolytic fungi in Vietnam reveals surprisingly high genetic diversity. Sci. Rep. 2019, 9, 3674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, I.; Powlowski, J.; Ishmael, N.; Darmond, C.; Marqueteau, S.; Moisan, M.-C.; Quenneville, G.; Tsang, A. A molecular phylogeny of thermophilic fungi. Fungal Biol. 2012, 116, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, V.; Kosarev, P.; Seledsov, I.; Vorobyev, D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006, 7 (Suppl. 1), S10. [Google Scholar] [CrossRef] [Green Version]
- Kalinowska, E.; Chodorska, M.; Paduch-Cichal, E.; Mroczkowska, K. An improved method for RNA isolation from plants using commercial extraction kits. Acta Biochim. Pol. 2012, 59, 391–393. [Google Scholar] [CrossRef] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savelli, B.; Li, Q.; Webber, M.; Jemmat, A.M.; Robitaille, A.; Zámocký, M.; Mathé, C.; Dunand, C. RedoxiBase: A database for ROS homeostasis regulated proteins. Redox Biol. 2019, 26, 101247. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediciton and analysis. Nat. Prot. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatsky, M.; Nussinov, R.; Wolfson, H.J. A method for simultaneous alignment of multiple protein structures. Proteins 2004, 56, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Dotsenko, G.; Tong, X.; Pilgaard, B.; Busk, P.K.; Lange, L. Acidic–alkaline ferulic acid esterase from Chaetomium thermophilum var. dissitum: Molecular cloning and characterization of recombinant enzyme expressed in Pichia pastoris. Biocatal. Agric. Biotechnol. 2016, 5, 48–55. [Google Scholar] [CrossRef]
- Amlacher, S.; Sarges, P.; Flemming, D.; van Noort, V.; Kunze, R.; Devos, D.P.; Arumugam, M.; Bork, P.; Hurt, E. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell 2011, 146, 277–289. [Google Scholar] [CrossRef] [Green Version]
- Bock, T.; Chen, W.H.; Ori, A.; Malik, N.; Silva-Martin, N.; Huerta-Cepas, J.; Powell, S.T.; Kastritis, P.L.; Smyshlyaev, G.; Vonkova, I.; et al. An integrated approach for genome annotation of the eukaryotic thermophile Chaetomium Thermophilum Nucl. Acids Res. 2014, 42, 13525–13533. [Google Scholar] [CrossRef] [Green Version]
- Zámocký, M.; Tafer, H.; Chovanová, K.; Lopandic, K.; Kamlárová, A.; Obinger, C. Genome sequence of the filamentous fungus Chaetomium cochliodes reveals abundance of genes for heme enzymes from all peroxidase and catalase superfamilies. BMC Genomics 2016, 17, 763. [Google Scholar] [CrossRef] [Green Version]
- Vetrovsky, T.; Kolarik, M.; Zifcakova, L.; Zelenka, T.; Baldrian, P. The rpb2 gene represents a viable alternative molecular marker for the analysis of environmental fungal communities. Mol. Ecol. Res. 2016, 16, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yang, H.; Ruan, L.; Liu, X.; Li, F.; Xu, X. Cloning and characterization of a thermostable superoxide dismutase from the thermophilic bacterium Rhodothermus sp. XMH10. J. Ind. Microbiol. Biotechnol. 2008, 35, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Robinett, N.G.; Peterson, R.L.; Culotta, V.C. Eukaryotic copper-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J. Biol. Chem. 2017, 293, 4636–4643. [Google Scholar] [CrossRef] [Green Version]
- Gessler, N.N.; Aver’yanov, A.A.; Belozerskaya, T.A. Reactive oxygen species in regulation of fungal development. Biochemistry (Mosc.) 2007, 72, 1091–1109. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Wang, W. Improving the thermostability and stress tolerance of an archaeon hyperthermophilic superoxide dismutase by fusion with a unique N-terminal domain. SpringerPlus 2016, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Kumar, P.; Lata, J.; Devi, N.; Chand, D. Thermostable Fe/Mn superoxide dismutase from Bacillus licheniformis SPB-13 from thermal springs of Himalayan region: Purification, characterization and antioxidative potential. Int. J. Biol. Macromol. 2018, 115, 1026–1032. [Google Scholar] [CrossRef]
- Norambuena, J.; Hanson, T.E.; Barkay, T.; Boyd, J.M. Superoxide dismutase and pseudocatalase increase tolerance to Hg (II) in Thermus thermophilus HB27 by maintaining the reduced bacillithiol pool. mBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.X.; Shi-Jin, E.; Liu, S.A.; Chen, J.; Li, D.C. Purification and characterization of a thermostable MnSOD from the thermophilic fungus Chaetomium thermophilum. Mycologia 2008, 100, 375–380. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Guo, F.X.; Xian, H.Q.; Wang, X.J.; Li, A.N.; Li, D.C. Expression of a novel thermostable Cu, Zn-superoxide dismutase from Chaetomium thermophilum in Pichia pastoris and its antioxidant properties. Biotechnol. Lett. 2011, 33, 1127–1132. [Google Scholar] [CrossRef]
- Gleason, J.A.; Galaleldeen, A.; Peterson, R.L.; Taylor, A.B.; Holloway, S.P.; Waninger-Saroni, J.; Cormack, B.P.; Cabelli, D.E.; Hart, P.J.; Cizewski Culotta, V. Candida albicans SOD5 represents the prototype of an unprecedented class of Cu-only superoxide dismutases required for pathogen defense. Proc. Natl. Acad. Sci. USA 2014, 111, 5866–5871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grum-Grzhimaylo, A.A.; Debets, A.J.M.; van Diepeningen, A.D.; Georgieva, M.L.; Bilanenko, E.N. Sodiomyces alkalinus, a new holomorphic alkaliphilic ascomycete within the Plectospaherellaceae. Persoonia 2013, 31, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haikarainen, T.; Frioux, C.; Zhang, L.-Q.; Li, D.-C.; Papageorgiou, A.C. Crystal structure and biochemical characterization of a manganese superoxide dismutase from Chaetomium thermophilum. Biochim. Biophys. Acta 2014, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Zámocký, M.; Gasselhuber, B.; Furtmüller, P.G.; Obinger, C. Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 2012, 525, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Zámocký, M.; Kamlárová, A.; Maresch, D.; Chovanová, K.; Harichová, J.; Furtmüller, P.G. Hybrid Heme Peroxidases from Rice Blast Fungus Magnaporthe oryzae Involved in Defence against Oxidative Stress. Antioxidants 2020, 9, 655. [Google Scholar] [CrossRef]
- Gennaro, P.; Bargna, A.; Bruno, F.; Sello, G. Purification of recombinant catalase-peroxidase HPI from E. coli and its application in enzymatic polymerization reactions. Appl. Microbiol. Biotechnol. 2014, 98, 1119–1126. [Google Scholar] [CrossRef]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and activators of SOD, GSH-Px, and CAT. In Enzyme Inhibitors and Activators; Senturk, M., Ed.; InTech: Croatia, Rijeka, 2017; pp. 207–224. ISBN 978-953-51-3058-1. [Google Scholar] [CrossRef] [Green Version]
- Sibirny, A.A. Yeast peroxisomes: Structure, functions and biotechnological opportunities. FEMS Yeast Res. 2016, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, A.; Manzoku, M.; Fukui, K.; Shimada, A.; Morita, R.; Masui, R.; Kuramitsu, S. Roles of Mn-catalase and a possible heme peroxidase homologue in protection from oxidative stress in Thermus thermophilus. Extremophiles 2015, 19, 775–785. [Google Scholar] [CrossRef]
- Jia, X.; Chen, J.; Lin, C.; Lin, X. Cloning, Expression, and Characterization of a Novel Thermophilic Monofunctional Catalase from Geobacillus sp. CHB1. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Shaeer, A.; Aslam, M.; Rashid, N. A highly stable manganese catalase from Geobacillus thermopakistaniensis: Molecular cloning and characterization. Extremophiles 2019, 23, 707. [Google Scholar] [CrossRef]
- Qi, H.; Wang, W.; He, J.; Ma, Y.; Xiao, F.; He, Y. Antioxidative system of Deinococcus radiodurans. Res. Microbiol. 2019, 171, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Chovanová, K.; Kamlárová, A.; Maresch, D.; Harichová, J.; Zámocký, M. Expression of extracellular peroxidases and catalases in mesophilic and thermophilic Chaetomia in response to environmental oxidative stress stimuli. Ecotox. Environ. Saf. 2019, 181, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Klotz, M.G.; Loewen, P.C. The molecular evolution of catalatic hydroperoxidases: Evidence for multiple lateral transfer of genes between prokaryota and from bacteria into eukaryota. Mol. Biol. Evol. 2003, 20, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.; Yainoy, S.; Prachayaitikul, V.; Büllow, L. A superoxide dismutase—Human hemoglobin fusion protein showing enhanced antioxidative properties. FEBS J. 2009, 276, 6195–6203. [Google Scholar] [CrossRef]







| Gene | NODE No., NG1-Length | NG1-Gene Position:START | NG1-Gene Position:END | NG1-Segment Length [bp] | NODE No., NG2-Length | NG2-Gene Position:START | NG2-Gene Position:END | NG2-Segment Length [bp] |
|---|---|---|---|---|---|---|---|---|
| 18S-5.8S-28S | NODE 965, 8730bp | 2845 | 8481 | 5637 | NODE 1882, 4524bp NODE 3062, 3145bp | 1800 2671 | 4524 5637 | 2725 2967 |
| Tubulin | NODE 244, 16828bp | 10315 | 12185 | 1871 | NODE 481, 8751bp | 2626 | 4496 | 1871 |
| Cthedis_rpb2 | NODE 1953, 4913bp | 1825 | 4913 | 3089 * | NODE 476, 8765bp | 5597 | 8734 | 3138 |
| CthediskatG1 | NODE 890, 9086bp | 1133 | 3743 | 2611 | NODE 2179, 4103bp | 1059 | 3669 | 2611 |
| Cthediskat2 | NODE 1031, 8272bp | 4055 | 7080 | 3026 | NODE 785, 7192bp | 1345 | 4370 | 3026 |
| Cthediskat3 | NODE 1987, 4805bp NODE 1207, 7396bp | 3369 1 | 4805 949 | 1437 949 | NODE 933, 6623bp | 1062 | 3392 | 2331 |
| CthedishyBpox1 | NODE, 1039, 8260bp | 4218 | 7654 | 3437 | NODE 1837, 4610bp | 430 | 3866 | 3437 |
| CthedisCuZnSOD1 | NODE 1294, 7102bp | 915 | 1809 | 895 | NODE 538, 8395bp | 2187 | 3081 | 895 |
| CthedisCuZnSOD2 | NODE 986, 8616bp | 1 | 952 | 952 * | NODE 3860, 2496bp NODE 1131, 6027bp | 49 5957 | 881 6027 | 833 * 71 * |
| CthedisFeSOD | NODE 3217, 2500bp | 748 | 1742 | 995 | NODE 4012, 2388bp | 659 | 1653 | 995 |
| CthedisMnSOD | NODE 15, 36954bp | 10874 | 11747 | 874 | NODE 769, 7230bp | 4132 | 5005 | 874 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chovanová, K.; Böhmer, M.; Poljovka, A.; Budiš, J.; Harichová, J.; Szemeš, T.; Zámocký, M. Parallel Molecular Evolution of Catalases and Superoxide Dismutases—Focus on Thermophilic Fungal Genomes. Antioxidants 2020, 9, 1047. https://doi.org/10.3390/antiox9111047
Chovanová K, Böhmer M, Poljovka A, Budiš J, Harichová J, Szemeš T, Zámocký M. Parallel Molecular Evolution of Catalases and Superoxide Dismutases—Focus on Thermophilic Fungal Genomes. Antioxidants. 2020; 9(11):1047. https://doi.org/10.3390/antiox9111047
Chicago/Turabian StyleChovanová, Katarína, Miroslav Böhmer, Andrej Poljovka, Jaroslav Budiš, Jana Harichová, Tomáš Szemeš, and Marcel Zámocký. 2020. "Parallel Molecular Evolution of Catalases and Superoxide Dismutases—Focus on Thermophilic Fungal Genomes" Antioxidants 9, no. 11: 1047. https://doi.org/10.3390/antiox9111047
APA StyleChovanová, K., Böhmer, M., Poljovka, A., Budiš, J., Harichová, J., Szemeš, T., & Zámocký, M. (2020). Parallel Molecular Evolution of Catalases and Superoxide Dismutases—Focus on Thermophilic Fungal Genomes. Antioxidants, 9(11), 1047. https://doi.org/10.3390/antiox9111047