Antioxidant Effects of Hemp (Cannabis sativa L.) Inflorescence Extract in Stripped Linseed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
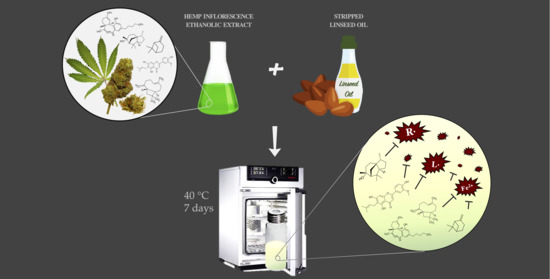
2.2. Preparation of the Ethanolic Extract
2.3. Characterization of Hemp Inflorescence Extract
2.3.1. Terpene Profile and Cannabidiol (CBD) Content
2.3.2. Total Phenolic Content (TPC)
2.3.3. Radical-Scavenging Activity (RSA)
2.3.4. Metal Ion-Chelating Activity
2.4. Preparation of Stripped Linseed Oil
2.5. Bulk Oil Model System: Sample Preparation and Storage Conditions
2.6. Evaluation of Oxidative Stability of Bulk Oil
2.6.1. Determination of Main Lipid Classes
2.6.2. Total Fatty Acid Composition
2.6.3. Determination of Peroxide Value
2.6.4. Determination of Total Volatile Compounds (VOCs)
2.7. Statistical Analysis
3. Results
3.1. Characterization of Hemp Inflorescence Extract
3.1.1. Total Phenolic Content (TPC), Terpene Profile, and Cannabidiol Content
3.1.2. Antioxidant Activity
3.2. Evaluation of the Oxidative Stability of Bulk Oil
3.2.1. Main Lipid Classes
3.2.2. Total Fatty Acid Composition
3.2.3. Peroxide Value (PV)
3.2.4. Volatile Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic review on N-3 and N-6 polyunsaturated fatty acid intake in European countries in light of the current recommendations—Focus on specific population groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, M.; Cardenia, V.; Rodriguez-Estrada, M.T.; Hrelia, S. Nutraceuticals and physical activity: Their role on oxysterols-mediated neurodegeneration. J. Steroid Biochem. Mol. Biol. 2019, 193, 105430. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Eur. Food Saf. Auth. J. 2013, 11, 1–103. [Google Scholar] [CrossRef] [Green Version]
- Harwood, J.L. Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Waraho, T.; Cardenia, V.; Rodriguez-Estrada, M.T.; McClements, D.J.; Decker, E.A. Prooxidant mechanisms of free fatty acids in stripped soybean oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 7112–7117. [Google Scholar] [CrossRef]
- Spitalniak-Bajerska, K.; Szumny, A.; Kucharska, A.Z.; Kupczyński, R. Effect of natural antioxidants on the stability of linseed oil and fish stored under anaerobic conditions. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Frankel, E.N. Free radical oxidation. In Lipid Oxidation, 2nd ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 15–24. ISBN 9780857097927. [Google Scholar]
- Rudzińska, M.; Przybylski, R.; Wąsowicz, E. Degradation of phytosterols during storage of enriched margarines. Food Chem. 2014, 142, 294–298. [Google Scholar] [CrossRef]
- Lanza, B.; Ninfali, P. Antioxidants in extra virgin olive oil and table olives: Connections between agriculture and processing for health choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, S.; Nishino, K.; Oikawa, S.; Inoue, S.; Mizutani, T.; Kawanishi, S. Oxidative DNA damage and apoptosis induced by metabolites of butylated hydroxytoluene. Biochem. Pharmacol. 1998, 56, 361–370. [Google Scholar] [CrossRef]
- Eskandani, M.; Hamishehkar, H.; Dolatabadi, J.E.N. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014, 153, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Szatkowska, K. Fruit and herbal meads—Chemical composition and antioxidant properties. Food Chem. 2019, 283, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Yu, R.; Li, J.; Wang, N.; Wang, Y.; Niu, L.; Zhang, Y. Application of several novel natural antioxidants to inhibit oxidation of tree peony seed oil. CyTA J. Food 2018, 16, 1071–1078. [Google Scholar] [CrossRef]
- Tura, M.; Mandrioli, M.; Gallina Toschi, T. Preliminary study: Comparison of antioxidant activity of cannabidiol (CBD) and α-tocopherol added to refined olive and sunflower oils. Molecules 2019, 24, 3485. [Google Scholar] [CrossRef] [Green Version]
- Cardenia, V.; Gallina Toschi, T.; Scappini, S.; Rubino, R.C.; Rodriguez-Estrada, M.T. Development and validation of a fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018, 26, 1283–1292. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic compounds and antioxidant activity of cold-pressed seed oil from finola cultivar of Cannabis sativa L. Phytother. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef]
- Lesma, G.; Consonni, R.; Gambaro, V.; Remuzzi, C.; Roda, G.; Silvani, A.; Vece, V.; Visconti, G.L. Cannabinoid-free Cannabis sativa L. grown in the Po valley: Evaluation of fatty acid profile, antioxidant capacity and metabolic content. Nat. Prod. Res. 2014, 28, 1801–1807. [Google Scholar] [CrossRef]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of phenolic compounds in commercial Cannabis sativa L. Inflorescences using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakro, F.; Jedryczka, M.; Wielgusz, K.; Sgorbini, B.; Inchingolo, R.; Cardenia, V. Simultaneous determination of terpenes and cannabidiol in hemp (Cannabis sativa L.) by fast gas chromatography with flame ionization detection. J. Sep. Sci. 2020, 43, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed electric field assisted extraction of bioactive compounds from cocoa bean shell and coffee silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Yen, G.C.; Chung, D.Y. Antioxidant effects of extracts from Cassia tora L. prepared under different degrees of roasting on the oxidative damage to biomolecules. J. Agric. Food Chem. 1999, 47, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; Xu, Z.; Yue, X.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors affecting lycopene oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 1408–1414. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ-and δ-tocopherol) levels in plant oils. Flavour Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis and the additional notes in the Annex to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff. Off. J. Eur. Union 2002, 45, 14. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2002:128:TOC (accessed on 7 November 2020).
- Kamvissis, V.N.; Barbounis, E.G.; Megoulas, N.C.; Koupparis, M.A. A Novel photometric method for evaluation of the oxidative stability of virgin olive oils. J. AOAC Int. 2008, 91, 794–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Z.; Fu, S.G.; Wang, S.Y.; Yang, D.J.; Wu, Y.H.S.; Chen, Y.C. Effects of a natural antioxidant, polyphenol-rich rosemary (Rosmarinus officinalis L.) extract, on lipid stability of plant-derived omega-3 fatty-acid rich oil. LWT Food Sci. Technol. 2018, 89, 210–216. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Res. Int. 2019, 123, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drinić, Z.; Vidović, S.; Vladić, J.; Koren, A.; Kiprovski, B.; Sikora, V. Effect of extraction solvent on total polyphenols content and antioxidant activity of Cannabis sativa L. Lekovite Sirovine 2018, 38, 17–21. [Google Scholar] [CrossRef]
- Legge 2 dicembre 2016; n. 242. Disposizioni per la promozione della coltivazione e della filiera agroindustriale della canapa (16G00258) (GU n. 304 del 30/12/2016). Gazz. Uff. della Repubb. Ital. 2017. Available online: https://www.gazzettaufficiale.it/eli/id/2016/12/30/16G00258/sg (accessed on 7 August 2020).
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Cannabis sativa and hemp. In Nutraceuticals; Elsevier, Academic Press: London, UK, 2016; pp. 735–754. ISBN 9780128021477. [Google Scholar]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L. The anxiolytic effect of Juniperus virginiana L. essential oil and determination of its active constituents. Physiol. Behav. 2018, 189, 50–58. [Google Scholar] [CrossRef]
- Cantele, C.; Rojo-Poveda, O.; Bertolino, M.; Ghirardello, D.; Cardenia, V.; Barbosa-Pereira, L.; Zeppa, G. In vitro bioaccessibility and functional properties of phenolic compounds from enriched beverages based on cocoa bean shell. Foods 2020, 9, 715. [Google Scholar] [CrossRef]
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Manosroi, A.; Chankhampan, C.; Kietthanakorn, B.O.; Ruksiriwanich, W.; Chaikul, P.; Boonpisuttinant, K.; Sainakham, M.; Manosroi, W.; Tangjai, T.; Manosroi, J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019, 46, 180–195. [Google Scholar]
- Chen, T.; He, J.; Zhang, J.; Li, X.; Zhang, H.; Hao, J.; Li, L. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Food Chem. 2012, 134, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Hydroperoxide decomposition. In Lipid Oxidation, 2nd ed.; Woodhead Publishing: Philadelphia, PA, USA, 2012; pp. 67–98. ISBN 9780857097927. [Google Scholar]
- Malvis, A.; Šimon, P.; Dubaj, T.; Sládková, A.; Ház, A.; Jablonsky, M.; Sekretár, S.; Schmidt, Š.; Kreps, F.; Burčová, Z.; et al. Determination of the thermal oxidation stability and the kinetic parameters of commercial extra virgin olive oils from different varieties. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Davies, C. Lipolysis in lipid oxidation. In Understanding and Measuring the Shelf-Life of Food; Steele, R., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2004; pp. 142–161. ISBN 9781855739024. [Google Scholar]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Ibargoitia, M.L.; Guillén, M.D. Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1H nuclear magnetic resonance. Food Chem. 2018, 245, 312–323. [Google Scholar] [CrossRef]
- Buscato, M.H.M.; Müller, F.; Vetter, W.; Weiss, J.; Salminen, H. Furan fatty acids in enriched ω-3 fish oil: Oxidation kinetics with and without added monomethyl furan fatty acid as potential natural antioxidant. Food Chem. 2020, 327, 127087. [Google Scholar] [CrossRef]
- Gordon, M.H. Factors affecting lipid oxidation. In Understanding and Measuring the Shelf-Life of Food; Steele, R., Ed.; Woodhead Publishing: Philadelphia, PA, USA, 2004; pp. 128–141. ISBN 9781855739024. [Google Scholar]
- Ma, L.; Liu, G.; Cheng, W.; Liu, X.; Brennan, C.; Brennan, M.A.; Liu, H.; Wang, Q. The effect of heating on the formation of 4-hydroxy-2-hexenal and 4-hydroxy-2-nonenal in unsaturated vegetable oils: Evaluation of oxidation indicators. Food Chem. 2020, 321, 126603. [Google Scholar] [CrossRef]
- Huang, S.W.; Frankel, E.N.; German, J.B. Antioxidant activity of α-and γ-tocopherols in bulk oils and in oil-in-water emulsions. J. Agric. Food Chem. 1994, 42, 2108–2114. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-Diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P.; Saavedra-Molina, A.; López-Meza, J.E.; Ochoa-Zarzosa, A.; Garciglia, R.S. Antioxidant activity of the essential oil and its major terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag. 2018, 13, S875–S880. [Google Scholar] [CrossRef]
- Misharina, T.A.; Samusenko, A.L. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Appl. Biochem. Microbiol. 2008, 44, 438–442. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Hacke, A.C.M.; Lima, D.; de Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the antioxidant activity of Δ9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef] [PubMed]






| Day 0 | Day 0.25 | Day 1 | Day 2 | Day3 | Day 5 | Day 7 | Sig. | ||
|---|---|---|---|---|---|---|---|---|---|
| ∑ SFAs | CO | 10.19 ± 0.03 | 10.21 ± 0.05 | 10.20 ± 0.03 | 10.22 ± 0.03 | 10.51 ± 0.10 | 10.28 ± 0.04 Y | 10.30 ± 0.05 XY | n.s. |
| HO | 10.17 ± 0.02 | 10.15 ± 0.08 | 10.21 ± 0.04 | 10.33 ± 0.16 | 10.15 ± 0.22 | 10.22 ± 0.02 Y | 10.23 ± 0.08 Y | n.s. | |
| EO | 10.16 ± 0.00 d | 10.19 ± 0.02 d | 10.23 ± 0.03 cd | 10.28 ± 0.02 bc | 10.32 ± 0.04 b | 10.41 ± 0.05 aX | 10.42 ± 0.04 aX | ** | |
| Sig. | n.s. | n.s. | n.s. | n.s. | n.s. | * | * | ||
| ∑ MUFAs | CO | 17.82 ± 0.01 b | 17.81 ± 0.07 b | 17.85 ± 0.01 bY | 17.86 ± 0.04 bXY | 17.87 ± 0.01 abY | 17.90 ± 0.04 abY | 17.96 ± 0.05 aY | * |
| HO | 17.80 ± 0.00 | 17.77 ± 0.06 | 17.83 ± 0.01 Y | 17.80 ± 0.04 Y | 17.86 ± 0.01 Y | 17.82 ± 0.01 Y | 17.85 ± 0.04 Y | n.s. | |
| EO | 17.83 ± 0.00 d | 17.85 ± 0.00 d | 17.89 ± 0.01 cdX | 17.96 ± 0.02 cX | 18.05 ± 0.02 bX | 18.18 ± 0.06 aX | 18.16 ± 0.04 aX | *** | |
| Sig. | n.s. | n.s. | * | * | ** | ** | * | ||
| ∑ PUFAs | CO | 71.46 ± 0.03 a | 71.41 ± 0.10 a | 71.41 ± 0.03 a | 71.38 ± 0.08 ab | 71.05 ± 0.13 c | 71.27 ± 0.10 abX | 71.17 ± 0.11 bc XY | ** |
| HO | 71.47 ± 0.03 | 71.52 ± 0.15 | 71.41 ± 0.06 | 71.29 ± 0.19 | 71.43 ± 0.21 | 71.40 ± 0.04 X | 71.37 ± 0.13 X | n.s. | |
| EO | 71.46 ± 0.00 a | 71.41 ± 0.03 a | 71.32 ± 0.03 ab | 71.21 ± 0.05 bc | 71.05 ± 0.07 c | 70.83 ± 0.12 dY | 70.86 ± 0.08 dY | *** | |
| Sig. | n.s. | n.s. | n.s. | n.s. | n.s. | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantele, C.; Bertolino, M.; Bakro, F.; Giordano, M.; Jędryczka, M.; Cardenia, V. Antioxidant Effects of Hemp (Cannabis sativa L.) Inflorescence Extract in Stripped Linseed Oil. Antioxidants 2020, 9, 1131. https://doi.org/10.3390/antiox9111131
Cantele C, Bertolino M, Bakro F, Giordano M, Jędryczka M, Cardenia V. Antioxidant Effects of Hemp (Cannabis sativa L.) Inflorescence Extract in Stripped Linseed Oil. Antioxidants. 2020; 9(11):1131. https://doi.org/10.3390/antiox9111131
Chicago/Turabian StyleCantele, Carolina, Marta Bertolino, Fatema Bakro, Manuela Giordano, Małgorzata Jędryczka, and Vladimiro Cardenia. 2020. "Antioxidant Effects of Hemp (Cannabis sativa L.) Inflorescence Extract in Stripped Linseed Oil" Antioxidants 9, no. 11: 1131. https://doi.org/10.3390/antiox9111131
APA StyleCantele, C., Bertolino, M., Bakro, F., Giordano, M., Jędryczka, M., & Cardenia, V. (2020). Antioxidant Effects of Hemp (Cannabis sativa L.) Inflorescence Extract in Stripped Linseed Oil. Antioxidants, 9(11), 1131. https://doi.org/10.3390/antiox9111131