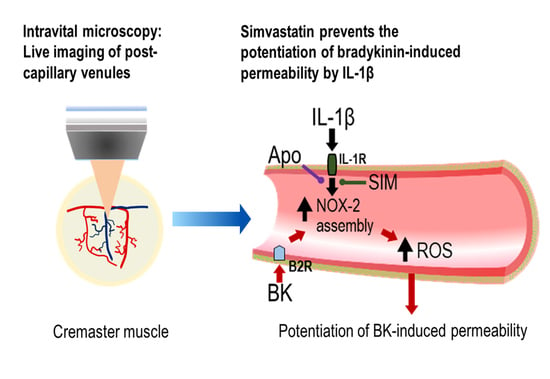
Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Isolation of the Cremaster Skeletal Muscle Preparation
2.2. Superfusion of Cremaster Muscle Preparation
2.3. Measurement of Post-Capillary Venule Permeability to FITC-Albumin
2.4. Role of Nitric Oxide and Reactive Oxygen Species in Microvascular Permeability
2.5. Bradykinin- and IL-1β-Induced Increases in Microvascular Permeability
2.6. Inhibition of NADPH Oxidase Assembly
2.7. Pretreatment of Animals with Simvastatin
2.8. Inhibition of Heme Oxygenase-1 with Tin Protophoryrin IX (SnPP)
2.9. Reagents
2.10. Statistical Analysis
3. Results
3.1. Role of NO and Reactive Oxygen Species in Modulating Basal Microvascular Permeability
3.2. Histamine- and Bradykinin-Induced Microvascular Permeability Is Mediated by Different Signaling Pathways
3.3. Bradykinin-Induced Microvascular Permeability Is Potentiated by IL-1β
3.4. A Role for NADPH Oxidase and Reactive Oxygen Species in the Potentiation of Bradykinin-Induced Microvascular Permeability by IL-1β
3.5. Pretreatment of Animals with Simvastatin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nussberger, J.; Cugno, M.; Cicardi, M. Bradykinin-mediated angioedema. N. Engl. J. Med. 2002, 347, 621–622. [Google Scholar] [CrossRef]
- Bas, M.; Adams, V.; Suvorava, T.; Niehues, T.; Hoffmann, T.K.; Kojda, G. Nonallergic angioedema: Role of bradykinin. Allergy 2007, 62, 842–856. [Google Scholar] [CrossRef]
- Obtulowicz, K. Bradykinin-mediated angioedema. Pol. Arch. Med. Wewn. 2016, 126, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Zausinger, S.; Lumenta, D.B.; Pruneau, D.; Schmid-Elsaesser, R.; Plesnila, N.; Baethmann, A. Effects of LF 16-0687 Ms, a bradykinin B(2) receptor antagonist, on brain edema formation and tissue damage in a rat model of temporary focal cerebral ischemia. Brain Res. 2002, 950, 268–278. [Google Scholar] [CrossRef]
- Ruiz, S.; Vardon-Bounes, F.; Buleon, M.; Guilbeau-Frugier, C.; Seguelas, M.H.; Conil, J.M.; Girolami, J.P.; Tack, I.; Minville, V. Kinin B1 receptor: A potential therapeutic target in sepsis-induced vascular hyperpermeability. J. Transl. Med. 2020, 18, 174. [Google Scholar] [CrossRef] [Green Version]
- Othman, R.; Vaucher, E.; Couture, R. Bradykinin Type 1 Receptor—Inducible Nitric Oxide Synthase: A New Axis Implicated in Diabetic Retinopathy. Front. Pharmacol. 2019, 10, 300. [Google Scholar] [CrossRef]
- Jacobson, J.R.; Barnard, J.W.; Grigoryev, D.N.; Ma, S.F.; Tuder, R.M.; Garcia, J.G. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L1026–L1032. [Google Scholar] [CrossRef]
- Nagaraja, T.N.; Knight, R.A.; Croxen, R.L.; Konda, K.P.; Fenstermacher, J.D. Acute neurovascular unit protection by simvastatin in transient cerebral ischemia. Neurol. Res. 2006, 28, 826–830. [Google Scholar] [CrossRef]
- Beziaud, T.; Ru Chen, X.; El Shafey, N.; Frechou, M.; Teng, F.; Palmier, B.; Beray-Berthat, V.; Soustrat, M.; Margaill, I.; Plotkine, M.; et al. Simvastatin in traumatic brain injury: Effect on brain edema mechanisms. Crit. Care Med. 2011, 39, 2300–2307. [Google Scholar] [CrossRef]
- Yang, D.; Knight, R.A.; Han, Y.; Karki, K.; Zhang, J.; Ding, C.; Chopp, M.; Seyfried, D.M. Vascular recovery promoted by atorvastatin and simvastatin after experimental intracerebral hemorrhage: Magnetic resonance imaging and histological study. J. Neurosurg. 2011, 114, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regoli, D. Neurohumoral regulation of precapillary vessels: The kallikrein-kinin system. J. Cardiovasc. Pharmacol. 1984, 6, S401–S412. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Roche, J.A.; Roche, R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 7265–7269. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Bruggemann, R.J.; van der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9, e57555. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Penninger, J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008, 93, 543–548. [Google Scholar] [CrossRef]
- Shimizu, S.; Ishii, M.; Yamamoto, T.; Kawanishi, T.; Momose, K.; Kuroiwa, Y. Bradykinin induces generation of reactive oxygen species in bovine aortic endothelial cells. Res. Commun. Chem. Pathol. Pharmacol. 1994, 84, 301–314. [Google Scholar]
- Woodfin, A.; Hu, D.E.; Sarker, M.; Kurokawa, T.; Fraser, P. Acute NADPH oxidase activation potentiates cerebrovascular permeability response to bradykinin in ischemia-reperfusion. Free Radic. Biol. Med. 2011, 50, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.H.; Hu, D.E.; Fraser, P.A. Acute effects of bradykinin on cerebral microvascular permeability in the anaesthetized rat. J. Physiol. 2000, 528, 177–187. [Google Scholar] [CrossRef]
- Sobey, C.G. Bradykinin B2 receptor antagonism: A new direction for acute stroke therapy? Br. J. Pharmacol. 2003, 139, 1369–1371. [Google Scholar] [CrossRef] [Green Version]
- Touzani, O.; Boutin, H.; Chuquet, J.; Rothwell, N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J. Neuroimmunol. 1999, 100, 203–215. [Google Scholar] [CrossRef]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e325–e331. [Google Scholar] [CrossRef]
- Ucciferri, C.; Auricchio, A.; Di Nicola, M.; Potere, N.; Abbate, A.; Cipollone, F.; Vecchiet, J.; Falasca, K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar]
- Stoll, L.L.; McCormick, M.L.; Denning, G.M.; Weintraub, N.L. Antioxidant effects of statins. Timely Top. Med. Cardiovasc. Dis. 2005, 9, E1. [Google Scholar] [CrossRef]
- Freitas, F.; Estato, V.; Reis, P.; Castro-Faria-Neto, H.C.; Carvalho, V.; Torres, R.; Lessa, M.A.; Tibirica, E. Acute simvastatin treatment restores cerebral functional capillary density and attenuates angiotensin II-induced microcirculatory changes in a model of primary hypertension. Microcirculation 2017, 24. [Google Scholar] [CrossRef]
- Margaritis, M.; Sanna, F.; Antoniades, C. Statins and oxidative stress in the cardiovascular system. Curr. Pharm. Des. 2017. [Google Scholar] [CrossRef]
- Ali, F.; Zakkar, M.; Karu, K.; Lidington, E.A.; Hamdulay, S.S.; Boyle, J.J.; Zloh, M.; Bauer, A.; Haskard, D.O.; Evans, P.C.; et al. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J. Biol. Chem. 2009, 284, 18882–18892. [Google Scholar] [CrossRef] [Green Version]
- Chartoumpekis, D.; Ziros, P.G.; Psyrogiannis, A.; Kyriazopoulou, V.; Papavassiliou, A.G.; Habeos, I.G. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2010, 396, 463–466. [Google Scholar] [CrossRef]
- Gueler, F.; Park, J.K.; Rong, S.; Kirsch, T.; Lindschau, C.; Zheng, W.; Elger, M.; Fiebeler, A.; Fliser, D.; Luft, F.C.; et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am. J. Pathol. 2007, 170, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.H.; Ko, W.J.; Hsu, J.Y.; Chen, J.S.; Lee, Y.C.; Lai, I.R.; Chen, C.F. Simvastatin ameliorates established pulmonary hypertension through a heme oxygenase-1 dependent pathway in rats. Respir. Res. 2009, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Makabe, S.; Takahashi, Y.; Watanabe, H.; Murakami, M.; Ohba, T.; Ito, H. Fluvastatin protects vascular smooth muscle cells against oxidative stress through the Nrf2-dependent antioxidant pathway. Atherosclerosis 2010, 213, 377–384. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Kopacz, A.; Kloska, D.; Zagrapan, B.; Neumayer, C.; Grochot-Przeczek, A.; Huk, I.; Brostjan, C.; Dulak, J.; Jozkowicz, A. Simvastatin Treatment Upregulates HO-1 in Patients with Abdominal Aortic Aneurysm but Independently of Nrf2. Oxidative Med. Cell. Longev. 2018, 2018, 2028936. [Google Scholar] [CrossRef] [Green Version]
- Chambers, D.J.; Fallouh, H.B. Cardioplegia and cardiac surgery: Pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacol. Ther. 2010, 127, 41–52. [Google Scholar] [CrossRef]
- Fraser, P.A.; Dallas, A.D.; Davies, S. Measurement of filtration coefficient in single cerebral microvessels of the frog. J. Physiol. 1990, 423, 343–361. [Google Scholar] [CrossRef]
- Ximenes, V.F.; Kanegae, M.P.; Rissato, S.R.; Galhiane, M.S. The oxidation of apocynin catalyzed by myeloperoxidase: Proposal for NADPH oxidase inhibition. Arch. Biochem. Biophys. 2007, 457, 134–141. [Google Scholar] [CrossRef]
- Kaizu, T.; Tamaki, T.; Tanaka, M.; Uchida, Y.; Tsuchihashi, S.; Kawamura, A.; Kakita, A. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003, 63, 1393–1403. [Google Scholar] [CrossRef] [Green Version]
- Cambridge, H.; Brain, S.D. Mechanism of bradykinin-induced plasma extravasation in the rat knee joint. Br. J. Pharmacol. 1995, 115, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Feletou, M.; Bonnardel, E.; Canet, E. Bradykinin and changes in microvascular permeability in the hamster cheek pouch: Role of nitric oxide. Br. J. Pharmacol. 1996, 118, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Holland, J.A.; Pritchard, K.A.; Pappolla, M.A.; Wolin, M.S.; Rogers, N.J.; Stemerman, M.B. Bradykinin induces superoxide anion release from human endothelial cells. J. Cell. Physiol. 1990, 143, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wambi-Kiesse, C.O.; Katusic, Z.S. Inhibition of copper/zinc superoxide dismutase impairs NO.-mediated endothelium-dependent relaxations. Am. J. Physiol. 1999, 276, H1043–H1048. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, S.; Ishida, S.; Gute, D.C.; Korthuis, R.J. Bradykinin-induced proinflammatory signaling mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2676–H2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Maejima, D.; Nagai, T.; Bridenbaugh, E.; Thangaswamy, S.; Chatterjee, V.; Meininger, C.J.; Gashev, A.A. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 2014, 21, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Manitsopoulos, N.; Orfanos, S.E.; Kotanidou, A.; Nikitopoulou, I.; Siempos, I.; Magkou, C.; Dimopoulou, I.; Zakynthinos, S.G.; Armaganidis, A.; Maniatis, N.A. Inhibition of HMGCoA reductase by simvastatin protects mice from injurious mechanical ventilation. Respir. Res. 2015, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Kao, M.C.; Shih, P.C.; Li, K.Y.; Tsai, P.S.; Huang, C.J. Simvastatin attenuates sepsis-induced blood-brain barrier integrity loss. J. Surg. Res. 2015, 194, 591–598. [Google Scholar] [CrossRef]
- Tuuminen, R.; Sahanne, S.; Loukovaara, S. Low intravitreal angiopoietin-2 and VEGF levels in vitrectomized diabetic patients with simvastatin treatment. Acta Ophthalmol. 2014, 92, 675–681. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, H. Simvastatin increases circulating endothelial progenitor cells and reduces the formation and progression of diabetic retinopathy in rats. Exp. Eye Res. 2012, 105, 1–8. [Google Scholar] [CrossRef]
- Caldwell, R.B.; Bartoli, M.; Behzadian, M.A.; El-Remessy, A.E.; Al-Shabrawey, M.; Platt, D.H.; Liou, G.I.; Caldwell, R.W. Vascular endothelial growth factor and diabetic retinopathy: Role of oxidative stress. Curr. Drug Targets 2005, 6, 511–524. [Google Scholar] [CrossRef]
- Dell’Omo, G.; Bandinelli, S.; Penno, G.; Pedrinelli, R.; Mariani, M. Simvastatin, capillary permeability, and acetylcholine-mediated vasomotion in atherosclerotic, hypercholesterolemic men. Clin. Pharmacol. Ther. 2000, 68, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuw Amerongen, G.P.; Vermeer, M.A.; Negre-Aminou, P.; Lankelma, J.; Emeis, J.J.; van Hinsbergh, V.W. Simvastatin improves disturbed endothelial barrier function. Circulation 2000, 102, 2803–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooradian, A.D.; Haas, M.J.; Batejko, O.; Hovsepyan, M.; Feman, S.S. Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes 2005, 54, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Liesmaa, I.; Kokkonen, J.O.; Kovanen, P.T.; Lindstedt, K.A. Lovastatin induces the expression of bradykinin type 2 receptors in cultured human coronary artery endothelial cells. J. Mol. Cell. Cardiol. 2007, 43, 593–600. [Google Scholar] [CrossRef]
- Blum, C.B. Comparison of properties of four inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Am. J. Cardiol. 1994, 73, 3d–11d. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Pleiotropic effects of statins—Basic research and clinical perspectives. Circ. J. Off. J. Jpn. Circ. Soc. 2010, 74, 818–826. [Google Scholar]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Kunz, M.; Nussberger, J.; Holtmannspötter, M.; Bitterling, H.; Plesnila, N.; Zausinger, S. Bradykinin in blood and cerebrospinal fluid after acute cerebral lesions: Correlations with cerebral edema and intracranial pressure. J. Neurotrauma 2013, 30, 1638–1644. [Google Scholar] [CrossRef]
- Dobrivojevic, M.; Spiranec, K.; Sindic, A. Involvement of bradykinin in brain edema development after ischemic stroke. Pflug. Arch. Eur. J. Physiol. 2015, 467, 201–212. [Google Scholar] [CrossRef]
- Eder, C. Mechanisms of interleukin-1beta release. Immunobiology 2009, 214, 543–553. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warboys, C.M.; Toh, H.B.; Fraser, P.A. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Ziff, M. Increased superoxide anion release from human endothelial cells in response to cytokines. J. Immunol. 1986, 137, 3295–3298. [Google Scholar] [PubMed]
- Wagner, A.H.; Kohler, T.; Ruckschloss, U.; Just, I.; Hecker, M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arter. Thromb. Vasc. Biol. 2000, 20, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Endres, M.; Laufs, U. Effects of statins on endothelium and signaling mechanisms. Stroke 2004, 35, 2708–2711. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Pendyala, S.; Natarajan, V.; Garcia, J.G.; Jacobson, J.R. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L575–L583. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, S.; Laufs, U.; Baumer, A.T.; Muller, K.; Konkol, C.; Sauer, H.; Bohm, M.; Nickenig, G. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: Involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol. Pharmacol. 2001, 59, 646–654. [Google Scholar] [CrossRef]
- Delbosc, S.; Morena, M.; Djouad, F.; Ledoucen, C.; Descomps, B.; Cristol, J.P. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J. Cardiovasc. Pharmacol. 2002, 40, 611–617. [Google Scholar] [CrossRef]
- Hordijk, P.L. Regulation of NADPH oxidases: The role of Rac proteins. Circ. Res. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Maack, C.; Kartes, T.; Kilter, H.; Schafers, H.J.; Nickenig, G.; Bohm, M.; Laufs, U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 2003, 108, 1567–1574. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Xue, Y. RhoA-mediated potential regulation of blood-tumor barrier permeability by bradykinin. J. Mol. Neurosci. 2010, 42, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wojciak-Stothard, B.; Potempa, S.; Eichholtz, T.; Ridley, A.J. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 2001, 114, 1343–1355. [Google Scholar] [PubMed]
- Boland, A.J.; Gangadharan, N.; Kavanagh, P.; Hemeryck, L.; Kieran, J.; Barry, M.; Walsh, P.T.; Lucitt, M. Simvastatin Suppresses Interleukin Iβ Release in Human Peripheral Blood Mononuclear Cells Stimulated With Cholesterol Crystals. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Miyazaki, T.; Hirano, M.; Akiyama, Y.; Mimura, T. Simvastatin inhibits production of interleukin 6 (IL-6) and IL-8 and cell proliferation induced by tumor necrosis factor-alpha in fibroblast-like synoviocytes from patients with rheumatoid arthritis. J. Rheumatol. 2006, 33, 463–471. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, F.; Tibiriçá, E.; Singh, M.; Fraser, P.A.; Mann, G.E. Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin. Antioxidants 2020, 9, 1269. https://doi.org/10.3390/antiox9121269
Freitas F, Tibiriçá E, Singh M, Fraser PA, Mann GE. Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin. Antioxidants. 2020; 9(12):1269. https://doi.org/10.3390/antiox9121269
Chicago/Turabian StyleFreitas, Felipe, Eduardo Tibiriçá, Mita Singh, Paul A. Fraser, and Giovanni E. Mann. 2020. "Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin" Antioxidants 9, no. 12: 1269. https://doi.org/10.3390/antiox9121269
APA StyleFreitas, F., Tibiriçá, E., Singh, M., Fraser, P. A., & Mann, G. E. (2020). Redox Regulation of Microvascular Permeability: IL-1β Potentiation of Bradykinin-Induced Permeability Is Prevented by Simvastatin. Antioxidants, 9(12), 1269. https://doi.org/10.3390/antiox9121269