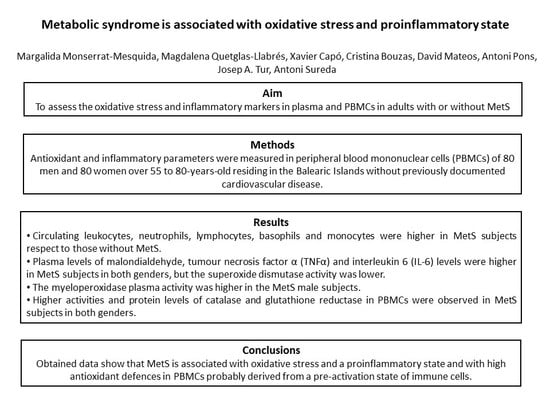
Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Anthropometric and Physical Activity Characterization
2.3. Blood Collection and Analysis
2.4. Cell Isolation and Cell Viability Test
2.5. Protein Levels
2.6. Enzymatic Determinations
2.7. Malondialdehyde Assay
2.8. Cytokines Assay
2.9. Statistics
3. Results
3.1. Anthropometric and Hematological Parameters
3.2. Oxidative Stress Biomarkers
3.3. Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic Syndrome and Risk of Incident Cardiovascular Events and Death. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, R.; Bibiloni, M.d.M.; Ramos, E.; Villarreal, J.Z.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome prevalence among Northern Mexican adult population. PLoS ONE 2014, 9, e105581. [Google Scholar] [CrossRef] [PubMed]
- Guallar-Castillón, P.; Pérez, R.F.; López García, E.; León-Muñoz, L.M.; Aguilera, M.T.; Graciani, A.; Gutiérrez-Fisac, J.L.; Banegas, J.R.; Rodríguez-Artalejo, F. Magnitud y manejo del síndrome metabólico en España en 2008-2010: Estudio ENRICA. Rev. Española Cardiol. 2014, 67, 367–373. [Google Scholar] [CrossRef]
- Beigh, S.H.; Jain, S. Prevalence of metabolic syndrome and gender differences. Bioinformation 2012, 8, 613–616. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Bekkouche, L.; Bouchenak, M.; Malaisse, W.J.; Yahia, D.A. The mediterranean diet adoption improves metabolic, oxidative, and inflammatory abnormalities in algerian metabolic syndrome patients. Horm. Metab. Res. 2014, 46, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Keaney, J.F. Role of Oxidative Modifications in Atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, C.; Xu, J.; Liechty, K.W. Targeting Inflammatory Cytokines and Extracellular Matrix Composition to Promote Wound Regeneration. Adv. Wound Care 2014, 3, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansyur, M.A.; Bakri, S.; Patellongi, I.J.; Rahman, I.A. The association between metabolic syndrome components, low-grade systemic inflammation and insulin resistance in non-diabetic Indonesian adolescent male. Clin. Nutr. ESPEN 2020, 35, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welty, F.K.; Alfaddagh, A.; Elajami, T.K. Targeting inflammation in metabolic syndrome. HHS Public Access 2016, 167, 257–280. [Google Scholar]
- Sheikhansari, G.; Soltani-Zangbar, M.S.; Pourmoghadam, Z.; Kamrani, A.; Azizi, R.; Aghebati-Maleki, L.; Danaii, S.; Koushaeian, L.; Hojat-Farsangi, M.; Yousefi, M. Oxidative stress, inflammatory settings, and microRNA regulation in the recurrent implantation failure patients with metabolic syndrome. Am. J. Reprod. Immunol. 2019, 82, 0–3. [Google Scholar] [CrossRef]
- Savaş, E.M.; Oğuz, S.H.; Samadi, A.; Yılmaz Işıkhan, S.; Ünlütürk, U.; Lay, İ.; Gürlek, A. Apoptosis Inhibitor of Macrophage, Monocyte Chemotactic Protein-1, and C-Reactive Protein Levels Are Increased in Patients with Metabolic Syndrome: A Pilot Study. Metab. Syndr. Relat. Disord. 2020. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care 2017, 5, e000379. [Google Scholar] [CrossRef]
- Morohoshi, M.; Fujisawa, K.; Uchimura, I.; Numano, F. The Effect of Glucose and Advanced Glycosylation End Products on IL-6 Production by Human Monocytes. Ann. N. Y. Acad. Sci. 2006, 748, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, D.; Ansorge, S. Elevated Glucose Levels Stimulate Transforming Growth Factor-β1 (TGF-β1), Suppress Interleukin IL-2, IL-6 and IL-10 Production and DNA Synthesis in Peripheral Blood Mononuclear Cells. Horm. Metab. Res. 1996, 28, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of Physical Activities: Classification of energy costs of human physical activities. Med. Sci. Sport. Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Bøyum, A. Separation of White Blood Cells. Nature 1964, 204, 793–794. [Google Scholar] [CrossRef]
- Mestre-Alfaro, A.; Ferrer, M.D.; Sureda, A.; Tauler, P.; Martínez, E.; Bibiloni, M.M.; Micol, V.; Tur, J.A.; Pons, A. Phytoestrogens enhance antioxidant enzymes after swimming exercise and modulate sex hormone plasma levels in female swimmers. Eur. J. Appl. Physiol. 2011, 111, 2281–2294. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training Enhances Immune Cells Mitochondrial Biosynthesis, Fission, Fusion, and Their Antioxidant Capabilities Synergistically with Dietary Docosahexaenoic Supplementation. Oxid. Med. Cell. Longev. 2016, 2016, 8950384. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar]
- Bergmayer, H.U. Glutathione reductase. Methods of Enzymatic Analysis. Starch - Stärke 1963, 15, 272. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [PubMed]
- Capeillère-Blandin, C. Oxidation of guaiacol by myeloperoxidase: A two-electron-oxidized guaiacol transient species as a mediator of NADPH oxidation. Biochem. J. 1998, 336, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmet, P.Z.; McCarty, D.J.; de Courten, M.P. The global epidemiology of non-insulin-dependent diabetes mellitus and the metabolic syndrome. J. Diabetes Complicat. 1997, 11, 60–68. [Google Scholar] [CrossRef]
- Bulló, M.; Casas-Agustench, P.; Amigó-Correig, P.; Aranceta, J.; Salas-Salvadó, J. Inflammation, obesity and comorbidities: The role of diet. Public Health Nutr. 2007, 10, 1164–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Eguilaz, M.H.R.; Batlle, M.A.; de Morentin, B.M.; San-Cristóbal, R.; Pérez-Díez, S.; Navas-Carretero, S.; Martínez, J.A. Cambios alimentarios y de estilo de vida como estrategia en la prevención del síndrome metabólico y la diabetes mellitus tipo 2: Hitos y perspectivas Alimentary and lifestyle changes as a strategy in the prevention. An. Sist. Sanit. Navar 2016, 39, 269–289. [Google Scholar]
- Nihi, M.M.; Manfro, R.C.; Martins, C.; Suliman, M.; Murayama, Y.; Riella, M.C.; Lindholm, B.; Nascimento, M.M. do [Association between body fat, inflammation and oxidative stress in hemodialysis]. J. Bras. Nefrol. 2010, 32, 9–15. [Google Scholar] [PubMed]
- Sureda, A.; Bibiloni, M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryder, E.; Diez-Ewald, M.; Mosquera, J.; Fernández, E.; Pedreañez, A.; Vargas, R.; Peña, C.; Fernández, N. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 8, 197–204. [Google Scholar] [CrossRef]
- Oda, E.; Kawai, R. The Prevalence of Metabolic Syndrome and Diabetes Increases through the Quartiles of White Blood Cell Count in Japanese Men and Women. Intern. Med. 2009, 48, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Li, P.-F.; Chen, J.-S.; Chang, J.-B.; Chang, H.-W.; Wu, C.-Z.; Chuang, T.-J.; Huang, C.-L.; Pei, D.; Hsieh, C.-H.; Chen, Y.-L. Association of complete blood cell counts with metabolic syndrome in an elderly population. BMC Geriatr. 2016, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Okon, E.B.; Chung, A.W.Y.; Zhang, H.; Laher, I.; van Breemen, C. Hyperglycemia and hyperlipidemia are associated with endothelial dysfunction during the development of type 2 diabetes. Can. J. Physiol. Pharmacol. 2007, 85, 562–567. [Google Scholar] [CrossRef]
- Rizzo, A.; Roscino, M.; Binetti, F.; Sciorsci, R. Roles of Reactive Oxygen Species in Female Reproduction. Reprod. Domest. Anim. 2012, 47, 344–352. [Google Scholar] [CrossRef]
- Sladoje, D.P.; Kisić, B.; Mirić, D. The Monitoring of Protein Markers of Inflammation and Serum Lipid Concentration in Obese Subjects with Metabolic Syndrome. J. Med. Biochem. 2017, 36, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Martorell, M.; Sureda, A.; Riera, J.; Drobnic, F.; Tur, J.A.; Pons, A. Effects of Almond- and Olive Oil-Based Docosahexaenoic- and Vitamin E-Enriched Beverage Dietary Supplementation on Inflammation Associated to Exercise and Age. Nutrients 2016, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Yen, C.H.; Huang, Y.C.; Lee, B.J.; Hsia, S.; Lin, P.T. Relationships between Inflammation, Adiponectin, and Oxidative Stress in Metabolic Syndrome. PLoS ONE 2012, 7, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-S.; Kor, C.-T.; Chen, T.-Y.; Liu, K.-H.; Shih, K.-L.; Su, W.-W.; Wu, H.-M. Relationships between Serum Uric Acid, Malondialdehyde Levels, and Carotid Intima-Media Thickness in the Patients with Metabolic Syndrome. Oxid. Med. Cell. Longev. 2019, 2019, 6859757. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Tauler, P.; Sureda, A.; Tur, J.A.; Pons, A. Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J. Sports Sci. 2009, 27, 49–58. [Google Scholar] [CrossRef]
- Hutcheson, R.; Rocic, P. The Metabolic Syndrome, Oxidative Stress, Environment, and Cardiovascular Disease: The Great Exploration. Exp. Diabetes Res. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ladeiras-Lopes, R.; Teixeira, P.; Azevedo, A.; Leite-Moreira, A.; Bettencourt, N.; Fontes-Carvalho, R. Metabolic syndrome severity score is associated with diastolic dysfunction and low-grade inflammation in a community-based cohort. Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef]
- Busquets-Cortés, C.; Capó, X.; del Mar Bibiloni, M.; Martorell, M.; Ferrer, M.D.; Argelich, E.; Bouzas, C.; Carreres, S.; Tur, J.A.; Pons, A.; et al. Peripheral blood mononuclear cells antioxidant adaptations to regular physical activity in elderly people. Nutrients 2018, 10, 1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capó, X.; Martorell, M.; Sureda, A.; Llompart, I.; Tur, J.A.; Pons, A. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur. J. Nutr. 2014, 54, 35–49. [Google Scholar] [CrossRef]
- Camargo, A.; Peña-Orihuela, P.; Rangel-Zúñiga, O.A.; Pérez-Martínez, P.; Delgado-Lista, J.; Cruz-Teno, C.; Marín, C.; Tinahones, F.; Malagón, M.M.; Roche, H.M.; et al. Peripheral blood mononuclear cells as in vivo model for dietary intervention induced systemic oxidative stress. Food Chem. Toxicol. 2014, 72, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, F.; Lattanzio, M.S.; Minetti, S.; Ansaldo, A.M.; Ferrara, D.; Molina-Molina, E.; Belfiore, A.; Elia, E.; Pugliese, S.; Palmieri, V.O.; et al. Circulating CRP Levels Are Associated with Epicardial and Visceral Fat Depots in Women with Metabolic Syndrome Criteria. Int. J. Mol. Sci. 2019, 20, 5981. [Google Scholar] [CrossRef] [Green Version]
- Jayarathne, S.; Koboziev, I.; Park, O.H.; Oldewage-Theron, W.; Shen, C.L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Sirota, P.; Hadi, E.; Djaldetti, M.; Bessler, H. Difference in inflammatory cytokine production by mononuclear cells from obese and non-obese schizophrenic patients. Acta Psychiatr. Scand. 2015, 132, 301–305. [Google Scholar] [CrossRef]
- Akhter, N.; Madhoun, A.; Arefanian, H.; Wilson, A.; Kochumon, S.; Thomas, R.; Shenouda, S.; Al-Mulla, F.; Ahmad, R.; Sindhu, S. Oxidative Stress Induces Expression of the Toll-Like Receptors (TLRs) 2 and 4 in the Human Peripheral Blood Mononuclear Cells: Implications for Metabolic Inflammation. Cell. Physiol. Biochem. 2019, 53, 1–18. [Google Scholar]



| Men | Women | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Without MetS (n = 40) | With MetS (n = 40) | Without MetS (n = 40) | With MetS (n = 40) | MetS | G | MetSxG | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||||
| Age (years) | 65.7 ± 0.8 | 63.8 ± 0.8 | 66.8 ± 0.8 | 64.1 ± 0.5 | 0.003 | 0.335 | 0.612 |
| Weight (kg) | 80.0 ± 1.7 *# | 93.4 ± 2.2 # | 64.0 ± 1.4 * | 77.1 ± 2.9 | <0.001 | <0.001 | 0.915 |
| Height (cm) | 170.0 ± 1.1 # | 169.3 ± 0.9 # | 156.4 ± 0.6 | 154.1 ± 0.9 | 0.102 | <0.001 | 0.379 |
| BMI (kg/m2) | 27.6 ± 0.5 * | 32.7 ± 0.7 | 26.1 ± 0.5 * | 33.3 ± 0.8 | <0.001 | 0.491 | 0.106 |
| Total physical activity (MET·hour/week) | 61.4 ± 3.5 a | 52.9 ± 6.9 a | 53.1 ± 2.3 a | 33.3 ± 4.8 b | 0.027 | <0.001 | 0.028 |
| Systolic blood pressure (mmHg) | 138.1 ± 3.4 | 138.8 ± 4.7 | 134.7 ± 2.4 | 127.4 ± 4.1 | 0.404 | 0.095 | 0.431 |
| Diastolic blood pressure (mmHg) | 81.6 ± 1.6 | 75.4 ± 2.4 | 79.1 ± 1.4 | 73.2 ± 2.3 | 0.003 | 0.265 | 0.868 |
| Glucose (mg/dL) | 97.8 ± 1.5 * | 120.9 ± 6.8 | 90.0 ± 1.2 * | 108.9 ± 4.6 | <0.001 | 0.012 | 0.486 |
| Triglycerides (mg/dL) | 95.0 ± 5.4 * | 153.5 ± 15.4 | 86.0 ± 4.7 * | 149.2 ± 10.4 | <0.001 | 0.464 | 0.865 |
| HDL-cholesterol (mg/dL) | 49.95 ± 1.20 a | 42.9 ± 1.60 b | 63.4 ± 1.47 c | 48.7 ± 1.34 a | <0.001 | <0.001 | 0.017 |
| Abdominal obesity (cm) | 95.0 ± 1.5 *# | 114.7 ± 1.8 # | 81.0 ± 1.2 * | 103.4 ± 3.2 | <0.001 | <0.001 | 0.359 |
| WHtR | 0.559 ± 0.010 * | 0.659 ± 0.020 | 0.518 ± 0.008 * | 0.671 ± 0.020 | <0.001 | 0.046 | 0.505 |
| Men | Women | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Without MetS (n = 40) | With MetS (n = 40) | Without MetS (n = 40) | With MetS (n = 40) | MetS | G | MetSxG | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||||
| Haematocrit (%) | 45.8 ± 0.54 # | 43.7 ± 0.78 | 41.5 ± 0.44 | 41.6 ± 0.4 | 0.103 | <0.001 | 0.091 |
| Erythrocytes (106/mm3) | 4.97 ± 0.06 a | 4.77 ± 0.09 a,b | 4.56 ± 0.05 b | 4.67 ± 0.07 b | 0.508 | <0.001 | 0.041 |
| Leukocytes (103/mm3) | 6.27 ± 0.24 * | 7.66 ± 0.30 | 5.63 ± 0.19 * | 7.22 ± 0.30 | <0.001 | 0.049 | 0.637 |
| Neutrophils (103/mm3) | 3.48 ± 0.20 *# | 4.36 ± 0.21 | 2.72 ± 0.12 * | 3.83 ± 0.19 | <0.001 | 0.001 | 0.461 |
| Lymphocytes (103/mm3) | 2.02 ± 0.08 | 2.27 ± 0.14 | 2.21 ± 0.11 | 2.52 ± 0.12 | 0.018 | 0.055 | 0.806 |
| Basophils (103/mm3) | 0.036 ± 0.004 * | 0.054 ± 0.005 | 0.040 ± 0.004 | 0.055 ± 0.004 | <0.001 | 0.321 | 0.950 |
| Monocytes (103/mm3) | 0.517 ± 0.021 * | 0.678 ± 0.036 | 0.435 ± 0.023 * | 0.596 ± 0.029 | <0.001 | 0.002 | 0.840 |
| Eosinophils (103/mm3) | 0.220 ± 0.032 | 0.238 ± 0.019 | 0.146 ± 0.012 | 0.217 ± 0.024 | 0.055 | 0.067 | 0.176 |
| Men | Women | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Without MetS (n = 40) | With MetS (n = 40) | Without MetS (n = 40) | With MetS (n = 40) | MetS | G | MetSxG | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||||
| Plasma activity | |||||||
| CAT (kat/L blood) | 84.4 ± 19.5 | 68.3 ± 6.7 | 123.6 ± 28.0 | 83.8 ± 14.8 | 0.118 | 0.116 | 0.548 |
| SOD (pkat/L sang) | 660.7 ± 44.7 * | 339.9 ± 23.3 | 689.5 ± 49.3 * | 331.4 ± 16.6 | <0.001 | 0.690 | 0.690 |
| MPO (μkat/mL blood) | 85.1 ± 25.4 * | 186.4 ± 22.2 | 90.1 ± 26.1 | 163.0 ± 23.9 | <0.001 | 0.614 | 0.478 |
| PBMCs activity | |||||||
| CAT (kat/109 cells) | 21.5 ± 4.63 * | 42.2 ± 4.94 | 22.4 ± 3.08 | 29.2 ± 3.18 | 0.001 | 0.145 | 0.096 |
| GRd (nkat/109 cells) | 211.5 ± 32.4 * | 408.2 ± 46.0 | 222.3 ± 38.3 * | 548.5 ± 55.7 | <0.001 | 0.114 | 0.177 |
| GPx (nkat/109 cells) | 56.0 ± 7.9 | 75.5 ± 5.8 | 54.9 ± 12.6 | 65.5 ± 6.3 | 0.059 | 0.496 | 0.590 |
| SOD (nkat/109 cells) | 72.3 ± 15.9 | 73.1 ± 5.61 | 48.1 ± 9.34 | 68.7 ± 6.10 | 0.242 | 0.123 | 0.280 |
| PBMCs protein levels | |||||||
| CAT (%) | 100.8 ± 6.6 * | 159.5 ± 10.5 | 100.0 ± 7.1 * | 130.1 ± 11.2 | <0.001 | 0.200 | 0.229 |
| GRd (%) | 90.4 ± 12.9 * | 132.6 ± 11.3 | 100.0 ± 11.8 * | 148.4 ± 15.9 | 0.001 | 0.398 | 0.877 |
| GPx (%) | 97.5 ± 6.6 | 96.5 ± 8.5 | 100.0 ± 8.8 | 113.9 ± 8.8 | 0.477 | 0.336 | 0.497 |
| MnSOD (%) | 97.0 ± 15.2 | 82.7 ± 17.7 | 100.0 ± 12.8 | 84.6 ± 8.42 | 0.293 | 0.864 | 0.967 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. https://doi.org/10.3390/antiox9030236
Monserrat-Mesquida M, Quetglas-Llabrés M, Capó X, Bouzas C, Mateos D, Pons A, Tur JA, Sureda A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants. 2020; 9(3):236. https://doi.org/10.3390/antiox9030236
Chicago/Turabian StyleMonserrat-Mesquida, Margalida, Magdalena Quetglas-Llabrés, Xavier Capó, Cristina Bouzas, David Mateos, Antoni Pons, Josep A. Tur, and Antoni Sureda. 2020. "Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State" Antioxidants 9, no. 3: 236. https://doi.org/10.3390/antiox9030236
APA StyleMonserrat-Mesquida, M., Quetglas-Llabrés, M., Capó, X., Bouzas, C., Mateos, D., Pons, A., Tur, J. A., & Sureda, A. (2020). Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants, 9(3), 236. https://doi.org/10.3390/antiox9030236