The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review
Abstract
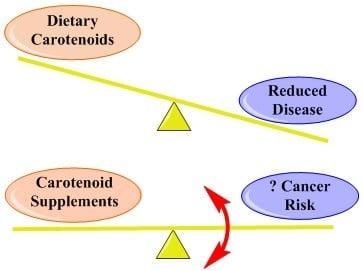
:1. Introduction
2. Epidemiological Studies and Clinical Trials
2.1. Porphyria
2.2. Cancer
2.2.1. Breast Cancer
2.2.2. Prostate Cancer
2.2.3. Colon Cancer
2.2.4. Skin Cancer
2.2.5. Lung Cancer
2.3. Age-Related Macular Degeneration (AMD)
3. Animal Studies
4. Molecular Mechanisms
4.1. Singlet Oxygen—General
4.2. Singlet Oxygen—Simple Solvents, Micelles and Liposomes
4.3. Singlet Oxygen—Ex Vivo Cell Studies
4.4. Singlet Oxygen—Porphyria
4.5. Singlet Oxygen—Cancers
4.6. Singlet Oxygen—Age-Related Macular Degeneration (AMD)
4.7. Radicals—General (In Vitro)
4.8. Radicals—Simple Solvents, Micelles and Liposomes
4.9. Radicals—Ex Vivo Cell Studies
4.10. Radicals—The Skin and EPP
4.11. Radicals—Eye Cancers and AMD
4.12. Radicals—Cancer
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Cancer-Preventive Agents. IARC Handbooks of Cancer Prevention: Carotenoids; IARC: Lyon, France, 1998. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Lazrak, T.; Milon, A.; Wolff, G.; Albrecht, A.-M.; Miehe, M.; Ourisson, G.; Natatani, Y. Comparison of the effects of inserted C40- and C50-terminally dihydroxylated carotenoids on the mechanical properties of various phospholipid vesicles. Biochim. Biophys. Acta 1987, 903, 132–141. [Google Scholar] [CrossRef]
- Bertram, J.S.; Vine, A.L. Cancer prevention by retinoids and carotenoids: Independent action on a common target. Biochim. Biophys. Acta 2005, 1740, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.M.; Stampfer, M.J.; Giovannucci, E.; Gann, P.H.; Ma, J.; Wilkinson, P. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science 1998, 279, 563–566. [Google Scholar] [CrossRef]
- Mathews, M.M. Protective effect of β-carotene against lethal photosensitization by haematoporphyrin. Nature 1964, 203, 1092. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Haber, L.H.; Kass, E.H. Beta-carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch. Dermatol. 1977, 113, 1229–1232. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Stukel, T.A.; Stevens, M.M.; Mandel, J.S.; Spencer, S.K.; Elias, P.M.; Lowe, N.; Nierenberg, D.W.; Bayrd, G.; et al. A clinical trial of β-carotene to prevent basal-cell and squamous-cell cancer of the skin. N. Engl. J. Med. 1990, 323, 789–795. [Google Scholar] [CrossRef]
- Rissanen, T.; Voutilainen, S.; Nyyssönen, K.; Lakka, T.A.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Low serum lycopene is associated with excess risk of acute coronary events and stroke: The Kuopio Ischaemic Risk Factor Study. Br. J. Nutr. 2001, 85, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Menon, I.A.; Persad, S.D.; Hasany, S.M.; Basu, P.K.; Becker, M.A.C.; Haberman, H.F. Reactive species involved in phototoxicity of photoporphyrin and uroporphyrin on bovine corneal endothelium. In Oxidative Damage and Repair, Proceedings of the 5th International Society for Free Radical Research, Bienial Meeting, Pasadena, CA, USA, 14–20 November 1990; Davies, K.J.A., Ed.; Pergamon Press: Oxford, UK, 1991; pp. 321–325. [Google Scholar]
- Menon, I.A.; Becker, M.A.; Persad, S.D.; Haberman, H.F. Quantitation of hydrogen peroxide formed during UV–visible irradiation of protoporphyrin, coproporphyrin and uroporphyrin. Clin. Chim. Acta 1990, 186, 375–381. [Google Scholar] [CrossRef]
- Pathak, M.A. Reactive oxygen species in inflammatory cutaneous photosensitivity reactions—A new insight. Clin. Exp. Dermatol. 1986, 11, 204. [Google Scholar]
- Mathews-Roth, M.M.; Pathak, M.A.; Fitzpatrick, T.B.; Harber, L.C.; Kass, E.H. Beta-carotene as a photoprotective agent in erythropoietic protoporphyria. N. Engl. J. Med. 1970, 282, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M. Carotenoids in erythropoietic protoporphyria and other photosensitivity diseases. Ann. N. Y. Acad. Sci. 1964, 691, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P.; Scharffetter, K.; Kind, P.; Goerz, G. Erythropoetische protoporphyrie: Synopsis von 20 patienten. Hautarzt 1991, 42, 570–574. [Google Scholar] [PubMed]
- Bohm, F.; Edge, R.; Foley, S.; Lange, L.; Truscott, T.G. Antioxidant inhibition of porphyrin-induced cellular phototoxicity. J. Photochem. Photobiol. B Biol. 2001, 65, 177–183. [Google Scholar] [CrossRef]
- Jansen, C.T. β-Carotene treatment of polymorphous light eruptions. Dermatologica 1974, 149, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Peto, R.; Doll, R.; Buckley, J.D.; Sporn, M.B. Can dietary β-carotene materially reduce human cancer rates? Nature 1981, 290, 201–208. [Google Scholar] [CrossRef]
- Shekelle, R.B.; Liu, S.; Raynor, W.J.; Leeper, M.; Maliza, C.; Rossof, A.H.; Pau, O.; Shryock, A.M.; Stamler, J. Dietary vitamin A and risk of cancer in the Western Electric study. Lancet 1981, 318, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Fairfield, K.M.; Fletcher, R.H. Vitamins for chronic disease prevention in adults. JAMA 2002, 287, 3116–3126. [Google Scholar] [CrossRef]
- Kune, G.A.; Bannerman, S.; Field, B.; Watson, L.F.; Cleland, H.; Merenstein, D.; Vitetta, L. Diet, alcohol, smoking, serum β-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr. Cancer 1992, 18, 237–244. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.-D.; Mucci, L.; Gaziano, J.M.; Zhang, S.M. Modulation of lung molecular biomarkers by β-carotene in the physicians’ health study. Cancer 2009, 115, 1049–1058. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, Y. Animal models in carotenoids research and lung cancer prevention. Transl. Oncol. 2011, 4, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costea, T.; Hudita, A.; Ciolac, O.-A.; Galateanu, B.; Ginghina, O.; Costache, M.; Ganea, C.; Mocanu, M.-M. Chemoprevention of colorectal cancer by dietary compounds. Int. J. Mol. Sci. 2018, 19, 3787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalabi, N.; Le Corre, L.; Maurizis, J.-C.; Bignon, Y.-J.; Bernard-Gallon, D.J. The effects of lycopene on the proliferation of human breast cells and BRCA1 and BRCA2 gene expression. Eur. J. Cancer 2004, 40, 1768–1775. [Google Scholar] [CrossRef]
- Gloria, N.F.; Soares, N.; Brand, C.; Olivera, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar]
- Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; Adami, H.O.; Beeson, W.L.; van den Brandt, P.A.; Folsom, A.R.; Fraser, G.E.; Freudenheim, J.L.; Goldbohm, R.A.; et al. Intake of fruits and vegetables and risk of breast cancer: A pooled analysis of cohort studies. JAMA 2001, 285, 769–776. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Navarro-Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Norat, T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: A systemic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2012, 96, 356–373. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.; Adams-Campbell, L.L.; Caan, B.J.; Chlebowski, R.T.; Neuhouser, M.L.; Shikany, J.M.; Rohan, T.E.; WHI Investigators. Longitudinal study of serum carotenoid, retinal, and tocopherol concentrations in relation to breast cancer risk among postmenopausal women. Am. J. Clin. Nutr. 2009, 90, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Maillard, V.; Kuriki, K.; Lefebvre, B.; Boutron-Ruault, M.C.; Lenoir, G.M.; Joulin, V.; Clavel-Chapelon, F.; Chales, V. Serum carotenoid, tocopherol and retinol concentrations and breast cancer risk in the E3N-EPIC study. Int. J. Cancer 2010, 127, 1188–1196. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Liao, X.; Rosner, B.; Tamimi, R.M.; Tworoger, S.S.; Hankinson, S.E. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am. J. Clin. Nutr. 2015, 101, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Glade, M.J. Food, nutrition, and the prevention of cancer: A general global perspective. Nutrition 1999, 15, 523–526. [Google Scholar] [PubMed]
- Ohno, Y.; Yoshida, O.; Oishi, K.; Okada, K.; Yamabe, H.; Schroeder, F.H. Dietary β-carotene and cancer of the prostate: A case-control study. Cancer Res. 1988, 48, 1331–1336. [Google Scholar] [PubMed]
- Wu, K.; Erdman, J.W.; Schwartz, S.J.; Platz, E.A.; Leitzmann, M.; Clintom, S.K.; DeGroff, V.; Willett, W.C.; Giovannucci, E. Plasma and dietary carotenoids and the risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A prospective study of tomato products, Lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Appleby, P.N.; Travis, R.C.; Albanes, D.; Alberg, A.J.; Barricarte, A.; Black, A.; Boeing, H.; Bueno-de-Mesquita, H.B.; Chan, J.M.; et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: Pooled analysis of 15 studies. Am. J. Clin. Nutr. 2015, 102, 1142–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.B.; Besterman-Dahan, K.; Kang, L.; Pow-Sang, J.; Xu, P.; Allen, K.; Riccardi, D.; Krischer, J.P. Results of a randomized clinical trial of the action of several doses of lycopene in localized prostate cancer: Administration prior to radical prostatectomy. Clin. Med. Urol. 2008, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of carotene and lycopene on the risk of prostate cancer: A systematic review and dose-Response meta-analysis of observational studies. PLoS ONE 2015, 10, e0137427. [Google Scholar] [CrossRef]
- Gontero, P.; Marra, G.; Soria, F.; Oderda, M.; Zitella, A.; Baratta, F.; Chiorino, G.; Gregnanin, I.; Daniele, L.; Cattel, L.; et al. A randomized double-blind placebo controlled phase I-II study of clinical and molecular effects of dietary supplements in men with precancerous prostatic lesions. Chemoprevention or “Chemopromotion”? Prostate 2015, 75, 1177–1186. [Google Scholar] [CrossRef]
- Barber, N.J.; Zhang, X.; Zhu, G.; Pramanik, R.; Barber, J.A.; Martin, F.L.; Morris, J.D.; Muir, G.H. Lycopene inhibits DNA synthesis in a primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis. 2006, 9, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Kucuk, O.; Sarkar, F.H.; Sakr, W.; Djuric, Z.; Pollak, M.N.; Khachik, F.; Li, Y.W.; Banerjee, M.; Grignon, D.; Bertram, J.S.; et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomark. Prev. 2001, 10, 861–868. [Google Scholar]
- Vance, T.A.; Azabdaftari, G.; Pop, E.A.; Lee, S.G.; Su, L.J.; Fontham, E.T.H.; Bensen, J.T.; Steck, S.E.; Arab, L.; Mohler, J.L.; et al. Intake of dietary antioxidants is inversely associated with biomarkers of oxidative stress among men with prostate cancer. Br. J. Nutr. 2016, 115, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Marques-Vidal, P.; Ravasco, P.; Ermelinda Camilo, M. Foodstuffs and colorectal cancer risk: A review. Clin. Nutr. 2006, 25, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A.; Ghadirian, P. Dietary carotenoids and risk of colon cancer: Case-control study. Int. J. Cancer 2004, 110, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Nomura, A.M.Y.; Murphy, S.P.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Carotenoid intake and colorectal cancer risk: The multi-ethnic cohort study. J. Epidemiol. 2009, 19, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Lu, M.-S.; Fang, Y.-J.; Xu, M.; Huang, W.Q.; Pan, Z.-Z.; Chen, Y.M.; Zhang, C.X. Serum carotenoids and colorectal cancer risk: A case-control study in Guandong, China. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Green, A.; Williams, G.; Neale, R.; Hart, V.; Leslie, D.; Parsons, P.; Marks, G.C.; Gaffney, P.; Battistuta, D.; Frost, C.; et al. A daily sunscreen application and beta carotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: A randomized trial. Lancet 1999, 354, 723–729. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Stukel, T.A.; Nierenberg, D.W.; Stevens, M.M.; Mandel, J.S.; Haile, R.W. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996, 275, 699–703. [Google Scholar] [CrossRef]
- Karagas, M.R.; Greenberg, E.R.; Nierenberg, D.; Stukel, T.A.; Morris, J.S.; Stevens, M.M.; Baron, J.A. Risk of squamous cell carcinoma of the skin in relation to plasma selenium, α-tocopherol, β-carotene, and retinol: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 1997, 6, 25–29. [Google Scholar]
- Stryker, W.S.; Stampfer, M.J.; Stein, E.A.; Kaplan, L.; Louis, T.A.; Sober, A.; Willett, W.C. Diet, plasma levels of β-carotene and alpha-tocopherol, and risk of malignant melanoma. Am. J. Epidemiol. 1990, 131, 597–611. [Google Scholar] [CrossRef]
- Kiripatrick, C.S.; White, E.; Lee, J.A.H. Case-control study of malignant melanoma in Washington State. Am. J. Epidemiol. 1994, 139, 869–880. [Google Scholar] [CrossRef]
- Breslow, R.A.; Alberg, A.J.; Helzlsouer, K.J.; Bush, T.L.; Norkus, E.P.; Morris, J.S.; Spate, V.E.; Comstock, G.W. Serological precursors of cancer: Malignant melanoma, basal and squamous cell skin cancer, and prediagnostic levels of retinol, β-carotene, lycopene, α-tocopherol, and selenium. Cancer Epidemiol. Biomark. Prev. 1995, 4, 837–842. [Google Scholar]
- Bayerl, C.; Schwarz, B.; Jung, E.G. Beneficial effect of β-carotene in dysplastic nevus syndrome—A randomized trial (abstract 068). In Proceedings of the 8th Congress of the European Society for Photobiology, Granada, Spain, 3–8 September 1999; p. 89. [Google Scholar]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barradon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-carotene supplementation and cancer risk: A systematic review and metaanalysis of randomized controlled trials. Int. J. Cancer 2010, 127, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Lambert, C.R. Radical reactions of carotenoids and potential influence of UV carcinogenesis. In Oxidants and Antioxidants in Cutaneous Biology; Thiele, J., Elsner, P., Eds.; Karger: Basel, Switzerland, 2001; Volume 29, pp. 140–156. [Google Scholar]
- The α-Tocopherol, β-Carotene Cancer Prevention Study Group. The effect of vitamin E and β-carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1996, 330, 1029–1035. [Google Scholar]
- Omenn, G.S. Chemoprevention of lung cancer: The rise and demise of beta-carotene. Ann. Rev. Public Health 1998, 19, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, C. Beta-carotene in dermatology: Does it help? Acta Dermatoven APA 2008, 17, 160–166. [Google Scholar]
- Landrum, J.T.; Bone, R.A.; Moore, L.L.; Gomez, C.M. Analysis of zeaxanthin distribution within individual human retinas. Methods Enzymol. 1999, 299, 457–467. [Google Scholar] [PubMed]
- Holz, F.G.; Pauleikhoff, D.; Spaide, R.F.; Bird, A.C. (Eds.) Age-Related Macular Degeneration; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Mathews-Roth, M.M.; Pathak, M.A.; Parrish, J.; Fitzpatrick, T.B.; Kass, E.H.; Toda, K.; Clemens, W. A clinical trial of the effects of beta-carotene on the responses of human skin to solar radiation. J. Investig. Dermatol. 1972, 59, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews-Roth, M.M.; Pathak, M.A. Phytoene as a protective agent against sunburn (>280 nm) radiation in guinea pigs. Photochem. Photobiol. 1975, 21, 261–263. [Google Scholar] [CrossRef]
- Epstein, J.H. Effects of β-carotene on UV-induced skin cancer formation in the hairless mouse skin. Photochem. Photobiol. 1977, 25, 211–213. [Google Scholar] [CrossRef]
- Santamaria, L.; Bianchi, A.; Arnaboldi, A.; Andreoni, L. Prevention of benzo(α)-pyrene photocarcinogenic effect by β-carotene and canthaxanthin. Med. Biol. Environ. 1981, 9, 113–120. [Google Scholar]
- Mathews-Roth, M.M. Antitumor activity of β-carotene, canthaxanthin, and phytoene. Oncology 1982, 38, 33–37. [Google Scholar] [CrossRef]
- Mathews-Roth, M.M. Carotenoid pigment administration and delay in development of UV-B-induced tumors. Photochem. Photobiol. 1983, 37, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.M.; Krinsky, N.I. Carotenoids affect development of UV-B induced skin cancer. Photochem. Photobiol. 1987, 46, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Mathews-Roth, M.M.; Krinsky, N.I. Carotenoid dose level and protection against UV-B- induced skin tumors. Photochem. Photobiol. 1985, 42, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.L.; Aickin, M.; Peng, Y.M. Cumulative reduction of primary skin tumor growth in UV-irradiated mice by the combination of retinyl palmitate and canthaxanthin. Cancer Lett. 1990, 53, 27–31. [Google Scholar] [CrossRef]
- Rybski, J.A.; Grogan, T.M.; Gensler, H.L. Reduction of murine cutaneous UVB-induced tumor-inflitrating T lymphocytes by dietary canthaxanthin. J. Investig. Dermatol. 1991, 97, 892–897. [Google Scholar] [CrossRef] [Green Version]
- Black, H.S. Radical interception by carotenoids and effects on UV carcinogenesis. Nutr. Cancer 1998, 31, 212–217. [Google Scholar] [CrossRef]
- Black, H.S.; Okotie-Eboh, G.; Gerguis, J. Aspects of β-carotene-potentiated photocarcinogenesis. Photochem. Photobiol. 1999, 69, 32S. [Google Scholar]
- Black, H.S. Reassessment of a free radical theory of cancer with emphasis on ultraviolet carcinogenesis. Integr. Cancer Ther. 2004, 3, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Black, H.S.; Okotie-Eboh, G.; Gerguis, J. Diet potentiates the UV-carcinogenic response to β-carotene. Nutr. Cancer 2000, 37, 173–178. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. β-carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar] [CrossRef]
- Nutrient Requirements of Laboratory Animals, 3rd ed.; Research Council/National Academy of Science: Washington, DC, USA, 1978; pp. 38–53.
- Black, H.S. Pro-carcinogenic activity of β-carotene, a putative systemic photoprotectant. Photochem. Photobiol. Sci. 2004, 3, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Gerguis, J. Modulation of dietary vitamins E and C fails to ameliorate β-carotene exacerbation of UV Carcinogenesis in mice. Nutr. Cancer 2003, 45, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S. Interaction of ascorbic acid and tocopherol on β-carotene modulated carcinogenesis. Hemoglobin 2010, 34, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Edge, R.; Truscott, T.G. β-carotene: Radical reactions and cancer associations – leading down a rabbit hole? J. Integr. Oncol. 2018, 7, 3. [Google Scholar] [CrossRef]
- Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; A Project of the World Cancer Research Fund/American Institute for Cancer Research (AICR): Washington, DC, USA, 2007.
- Kanofsky, J.R. Singlet oxygen production by biological systems. Chem. Biol. Interact. 1989, 70, 1–28. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Kröncke, K.-D.; Sies, H. Singlet oxygen-induced signalling effects in mammalian cells. Photochem. Photobiol. Sci. 2003, 2, 88–94. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Truscott, T.G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef]
- Oliveros, E.; Braun, A.M.; Aminian-Saghafi, T.; Sliwka, H.-R. Quenching of singlet oxygen (1Δg) by carotenoid derivatives: Kinetic analysis by near-infrared luminescence. New J. Chem. 1994, 18, 535–539. [Google Scholar]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as antioxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189–200. [Google Scholar] [CrossRef]
- Teraoka, J.; Hashimoto, H.; Matsudaira, S.; Koyama, Y. Resonance Raman spectra of excited triplet states of β-carotene isomers. Chem. Lett. 1985, 14, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Heymann, T.; Heinz, P.; Glomb, M.A. Lycopene inhibits the isomerization of β-carotene during quenching of singlet oxygen and free radicals. J. Agric. Food. Chem. 2015, 63, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Ouchi, A.; Azuma, N.; Takahashi, S.; Aizawa, K.; Nagaoka, S. Development of a singlet oxygen absorption capacity (SOAC) assay method. Measurements of the SOAC values for carotenoids and α-tocopherol in an aqueous Triton X-100 micellar solution. J. Agric. Food Chem. 2017, 65, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G. Singlet oxygen quenching by dietary carotenoids in a model membrance environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Fukuzawa, K. Singlet oxygen scavenging in phospholipid membranes. Methods Enzymol. 2000, 319, 101–110. [Google Scholar] [PubMed]
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and α-tocopherol in liposomes. Lipids 1998, 33, 751–756. [Google Scholar] [CrossRef]
- Lindig, B.A.; Rodgers, M.A.J. Rate parameters for the quenching of singlet oxygen by water-soluble and lipid-soluble substrates in aqueous and micellar systems. Photochem. Photobiol. 1981, 33, 627–634. [Google Scholar] [CrossRef]
- Edge, R. Spectroscopic and Kinetic Investigations of Carotenoid Radical Ions and Excited States. Ph.D. Thesis, Keele University, Keele, UK, 1998. [Google Scholar]
- Morita, M.; Naito, Y.; Yoshikawa, T.; Niki, E. Rapid assessment of singlet oxygen-induced plasma lipid oxidation and its inhibition by antioxidants with diphenyl-1-pyrenylphosphine (DPPP). Anal. Bioanal. Chem. 2016, 408, 265–270. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Böehm, F.; Haley, J.; Truscott, T.G.; Schalch, W. Cellular bound β-carotene quenches singlet oxygen in man. J. Photochem. Photobiol. B Biol. 1993, 21, 219–221. [Google Scholar] [CrossRef]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Tinkler, J.H.; Böhm, F.; Schalch, W.; Truscott, T.G. Dietary carotenoids protect human cells from damage. J. Photochem. Photobiol. B Biol. 1994, 26, 283–285. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Burke, M.; Truscott, T.G. Dietary uptake of lycopene protects human cells from singlet oxygen and nitrogen dioxide - ROS components from cigarette smoke. J. Photochem. Photobiol. B Biol. 2001, 64, 176–178. [Google Scholar] [CrossRef]
- Bosio, G.N.; Breitenbach, T.; Parisi, J.; Reigosa, M.; Blaikie, F.H.; Pedersen, B.W.; Silva, E.F.F.; Martire, D.O.; Ogilby, P.R. Antioxidant β-carotene does not quench singlet oxygen in mammalian cells. J. Am. Chem. Soc. 2013, 135, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.N.; Telfer, A.; Truscott, T.G.; Barber, J. Direct detection of singlet oxygen from isolated photosystem two reaction centres. Biochim. Biophys. Acta 1993, 1143, 301–309. [Google Scholar] [CrossRef]
- Harayama, O.; Nakamura, K.; Hamada, S.; Kobayasi, Y. Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 1994, 29, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Telfer, A. Singlet oxygen production by PSII under light stress: Mechanism, detection and the protective role of β-carotene. Plant Cell Physiol. 2014, 55, 1216–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telfer, A.; Dhami, S.; Bishop, S.M.; Phillips, D.; Barber, J. β-carotene quenches singlet oxygen formed by isolated photosystem II reaction centres. Biochemistry 1994, 33, 14469–14474. [Google Scholar] [CrossRef]
- Rossetti, F.C.; Depieri, L.V.; Tedesco, A.C.; Bentley, M.V.L.B. Fluorometric quantification of protoporphyrin IX in biological skin samples from in vitro penetration/permeation studies. Braz. J. Pharm. Sci. 2010, 46, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Palozza, P.; Krinsky, N.I. Antioxidant effects of carotenoids in vivo and in vitro: An overview. Methods Enzymol. 1992, 23, 403–422. [Google Scholar]
- Góralczyk, R. β-Carotene and lung cancer in smokers: Review of hypotheses and status of research. Nutr. Cancer 2009, 61, 767–774. [Google Scholar] [CrossRef]
- Schwarz, J.I.; Singh, R.P.; Teicher, B.; Wright, J.E.; Trites, D.H.; Shklar, G. Induction of a 70 kD protein associated with the selective cytotoxicity of beta-carotene in human epidermal carcinoma. Biochem. Biophys. Res. Commun. 1990, 29, 941–946. [Google Scholar] [CrossRef]
- Fung, T.T.; Spiegelman, D.; Egan, K.M.; Giovannucci, E.; Hunter, D.J.; Willett, W.C. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int. J. Cancer 2003, 103, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, J.P.; Michel, L.; Maurette, M.; Adhoute, H.; Bejot, M. Immediate effects of UV radiation on the skin: Modification by an antioxidant complex containing carotenoids. Photoderm. Photoimmunol. Photomed. 2003, 19, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeum, K.-J.; Beretta, G.; Krinsky, I.N.; Russell, R.M.; Aldini, G. Synergistic Interactions of Antioxidant Nutrients in a Biological Model system. Nutrition 2009, 25, 839–846. [Google Scholar] [CrossRef]
- Balić, A.; Mokos, M. Do we utilize our knowledge of the skin protective effects of carotenoids enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davinelli, S.; Nielsen, M.E.; Giovanni Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Juin, C.; de Olivera Junior, R.G.; Fleury, A.; Oudinet, C.; Pytowski, L.; Bérard, J.-B.; Nicolau, E.; Thiéry, V.; Lanneluc, I.; Beaugeard, L.; et al. Zeaxanthin from Porphtridium purpureum induces apoptosis in human melanoma cells expressing the oncogegnic BRAF V600E mutation and sensitizes them to the BRAF inhibitor vemurafenib. Rev. Bras. Farmacogn. 2018, 28, 457–467. [Google Scholar] [CrossRef]
- Wu, N.-L.; Chiang, Y.-C.; Huang, C.-C.; Fang, J.-Y.; Chen, D.-F.; Hung, C.-F. Zeaxanthin inhibits PDGF-BB-induced migration in human dermal fibroblasts. Exp. Dermatol. 2010, 19, e173–e181. [Google Scholar] [CrossRef]
- Suzukawaa, A.A.; Vieiraa, A.; Winnischofer, S.M.B.; Scalfob, A.C.; Di Mascio, P.; da Costa Ferreira, A.M.; Ravanat, J.-L.; de Luna Martinse, D.; Rocha, M.E.M.; Martinez, G.R. Novel properties of melanin include promotion of DNA strand breaks, impairment of repair, and reduced ability to damage DNA after quenching of singlet oxygen. Free Radic. Biol. Med. 2012, 52, 1945–1953. [Google Scholar] [CrossRef]
- Sarna, T.; Menon, I.A.; Sealy, R.C. Photosensitization of melanin: A comparative study. Photochem. Photobiol. 1985, 42, 529–532. [Google Scholar] [CrossRef]
- Rozanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free radical scavenging properties of melanin: Interaction of eu- and pheo-melanin models with reducing and oxidizing radicals. Free Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Brady, W.; Klein, R.; Klein, B.; Bowen, P.; Stacewiczsapuntzakis, M.; Palta, M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch. Ophthalmol. 1995, 113, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, F.; Zarrilli, F.; Mazzeo, S.; Verde, V.; Romano, N.; Savoia, M.; Testa, F.; Vitale, D.F.; Rinaldi, M.; Sacchetti, L. Serum oxidative and antioxidant parameters in a group of Italian patients with age-related maculopathy. Clin. Chim. Acta 2002, 320, 111–115. [Google Scholar] [CrossRef]
- Cardinault, N.; Abalain, J.-H.; Sairafi, B.; Coudray, C.; Grolier, P.; Rambeau, M.; Carré, J.-C. Lycopene but not lutein nor zeaxanthin decreases in serum and lipoproteins in age-related macular degeneration patients. Clin. Chim. Acta 2005, 357, 34–42. [Google Scholar] [CrossRef]
- Li, B.; Bernstein, P.S. Macular pigment carotenoids and their role in human eye health and diseases. In Studies on Retinal and Choridal Disorders, Oxidative Stress in Applied Basic Research and Clinical Practice; Stratton, R.D., Hauswirth, W.W., Gardner, T.W., Eds.; Springer: New York, NY, USA, 2012; Chapter 31; pp. 613–627. [Google Scholar]
- Galano, A.; Vargas, V.; Martinez, A. Carotenoids can act as antioxidants by oxidising the superoxide radical anion. Phys. Chem. Chem. Phys. 2010, 12, 193–200. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Truscott, T.G.; Witt, C. A dramatic effect of oxygen on protection of human cells against ɣ-radiation by lycopene. FEBS Lett. 2016, 590, 1086–1093. [Google Scholar] [CrossRef] [Green Version]
- Land, E.J.; Lafferty, J.; Roach, A.C.; Sinclair, R.S.; Truscott, T.G. Absorption spectra of radical ions of polyenes of biological interest. J. Chem. Soc. Faraday Trans. 1977, 73, 416–429. [Google Scholar]
- Edge, R.; Land, E.J.; McGarvey, D.J.; Burke, M.; Truscott, T.G. The reduction potential of the β-carotene•+/ β-carotene couple in an aqueous micro-heterogeneous environment. FEBS Lett. 2000, 471, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Burke, M.; Edge, R.; Land, E.J.; McGarvey, D.J.; Truscott, T.G. One-electron reduction potentials of dietary carotenoid radical cations in aqueous micellar environments. FEBS Lett. 2001, 500, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Tinkler, J.H.; Tavender, S.M.; Parker, A.W.; McGarvey, D.J.; Mulroy, L.; Truscott, T.G. An investigation of carotenoid radical cations and triplet states by laser flash photolysis and time-resolved resonance Raman spectroscopy: Observation of competitive energy and electron transfer. J. Am. Chem. Soc. 1996, 118, 1756–1761. [Google Scholar] [CrossRef]
- Cheng, H.; Han, R.-M.; Lyu, M.-K.; Zhang, J.-P.; Skibsted, L.H. Regeneration of β-carotene from the radical cation by tyrosine and tryptophan. J. Phys. Chem. B 2015, 119, 6603–6610. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-T.; Cheng, H.; Han, R.-M.; Wang, P.; Zhang, J.-P.; Skibsted, L.H. Regeneration of β-carotene from radical cation by eugenol, isoeugenol, and clove oil in the Marcus theory inverted region for electron transfer. J. Agric. Food Chem. 2017, 65, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Mairanovsky, V.G.; Engovatov, A.A.; Ioffe, N.T.; Samokhvalov, G.I. Electron-donor and electron-acceptor properties of carotenoids: Electrochemical study of carotenes. J. Electroanal. Chem. 1975, 66, 122–137. [Google Scholar] [CrossRef]
- Focsan, A.L.; Pan, S.; Kispert, L.D. Electrochemical study of astaxanthin and astaxanthinn-octanoic monoester and diester: Tendency to form radicals. J. Phys. Chem. B 2014, 118, 2331–3229. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Grant, J.L.; Metzger, R.M.; Kispert, L.D. Carotenoid radical cations produced by the interaction of carotenoids with iodine. J. Am. Chem. Soc. 1988, 92, 4600–4606. [Google Scholar] [CrossRef]
- Edge, R.; El-Agamey, A.; Land, E.J.; Navaratnam, S.; Truscott, T.G. Studies of carotenoid one-electron reduction radicals. Arch. Biochem. Biophys. 2007, 458, 104–110. [Google Scholar] [CrossRef]
- Edge, R.; Land, E.J.; McGarvey, D.; Mulroy, L.; Truscott, T.G. Relative one- electron reduction potentials of carotenoid radical cations and interactions of carotenoids with the vitamin E radical cation. J. Am. Chem. Soc. 1998, 120, 4087–4090. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Land, E.J.; McGarvey, D.J.; Truscott, T.G. Carotenoids enhance vitamin E antioxidant efficiency. J. Am. Chem. Soc. 1997, 119, 621–622. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of lycopene as an antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet oxygen and free radical reactions of retinoids and carotenoids—A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Hill, T.J.; Land, E.J.; McGarvey, D.J.; Schalch, W.; Tinkler, J.H.; Truscott, T.G. Interactions between carotenoids and the CCl3O2• radical. J. Am. Chem. Soc. 1995, 117, 8322–8326. [Google Scholar] [CrossRef]
- Edge, R. Radiolytic and Photolytic Production of Free Radicals and Reactive Oxygen Species: Interactions with Antioxidants and Biomolecules. In Applied Photochemistry; Evans, R., Douglas, P., Burrow, H., Eds.; Springer: Dordrecht, The Netherlands, 2013; Chapter 8; pp. 305–330. [Google Scholar]
- Halliwall, B.; Gutteridge, J.M.C. Role of free radicals in human disease: An overview. In Methods in Enzymology, Vol 186 Oxygen Radicals in Biological Systems, Part B Oxygen Radicals and Antioxidants; Academic Press: San Diego, CA, USA, 2000; pp. 1–85. [Google Scholar]
- Edge, R.; Land, E.J.; Rozanowska, M.; Sarna, T.; Truscott, T.G. Carotenoid radical – melanin interactions. J. Phys. Chem. B 2000, 104, 7193–7196. [Google Scholar] [CrossRef]
- Rock, C.L. Relationship of Carotenoids to Cancer. In Carotenoids in Health and Disease; Krinsky, N.I., Mayne, S.T., Sies, H., Eds.; Marcel Dekker: New York, NY, USA, 2004; Chapter 17; pp. 373–407. [Google Scholar]
- Anderson, H.J.; Chen, H.; Pellet, L.J.; Tappel, A.L. Ferrous-iron-induced oxidation in chicken liver slices as measured by hemichrome formation and thiobarbituric acid-reactive substances: Effects of dietary vitamin E and beta-carotene. Free Radic. Biol. Med. 1993, 15, 37–48. [Google Scholar] [CrossRef]
- Truscott, T.G. β-carotene and disease: A suggested pro-oxidant and antioxidant mechanism and speculations concerning its role in cigarette smoking. J. Photochem. Photobiol. B 1996, 35, 233–235. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Carotenoids: Free radical reactions. Spectrum 2000, 13, 12–20. [Google Scholar]







| Diet | Median Tumor Time, Weeks | Tumors/Animal |
|---|---|---|
| Closed formula | ||
| Control | 20.6 | 0.52 |
| 0.07% β-Carotene | 20.0 | 0.60 |
| Semi-defined | ||
| Control | 19.5 | 0.60 |
| 0.07% β-Carotene | 17.2 | 1.63 |
| Control (−βCar) a | (+βCar) b | (+βCar, −Vit C) c | (+βCar, −Vit C, low Vit E) d |
|---|---|---|---|
| 1.05 | 3.20 | 3.45 | 5.90 |
| Carotenoid | n | kq/109 dm3mol−1s−1 |
|---|---|---|
| lycopene | 11 | 17.0 |
| β-carotene | 11 | 13.0 |
| zeaxanthin | 11 | 12.6 |
| Meso-zeaxanthin | 11 | 12.2 |
| α-carotene | 10 | 12.0 |
| lutein | 10 | 6.64 |
| septapreno-β-carotene | 9 | 1.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. https://doi.org/10.3390/antiox9030264
Black HS, Boehm F, Edge R, Truscott TG. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants. 2020; 9(3):264. https://doi.org/10.3390/antiox9030264
Chicago/Turabian StyleBlack, Homer S., Fritz Boehm, Ruth Edge, and T. George Truscott. 2020. "The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review" Antioxidants 9, no. 3: 264. https://doi.org/10.3390/antiox9030264
APA StyleBlack, H. S., Boehm, F., Edge, R., & Truscott, T. G. (2020). The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants, 9(3), 264. https://doi.org/10.3390/antiox9030264