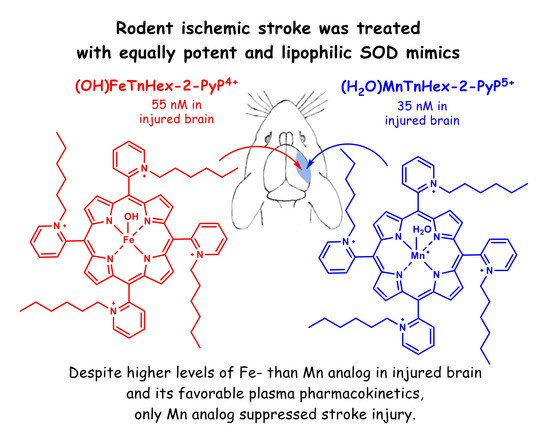
Fe Porphyrin-Based SOD Mimic and Redox-Active Compound, (OH)FeTnHex-2-PyP4+, in a Rodent Ischemic Stroke (MCAO) Model: Efficacy and Pharmacokinetics as Compared to Its Mn Analogue, (H2O)MnTnHex-2-PyP5+
Abstract
:1. Introduction
2. Experimental
3. Materials and Methods
3.1. Animals
3.2. LC-MS/MS Analysis of MnHex and FeHex
3.3. Pharmacokinetic (PK) Studies
3.3.1. The 3-Day PK Study in Healthy Mice
3.3.2. The 7-Day PK Study in Healthy Rats
3.3.3. The 2-h PK Study in Rats that Underwent MCAO
3.4. Efficacy MCAO Studies
3.4.1. The 3-Day Mouse MCAO Study
3.4.2. The 7-Day Rat MCAO Study
4. Results
4.1. Pharmacokinetic Studies
4.1.1. The 3-Day Mouse PK Study
4.1.2. The 7-Day Rat PK Study
4.1.3. The 2-h Rat PK Study in an MCAO Model
4.2. Efficacy Studies
4.2.1. The 3-Day Mouse MCAO Study
4.2.2. The 7-Day Rat MCAO Study
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Batinic-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins-From superoxide dismutation to H2O2-driven pathways. Redox Biol. 2015, 5, 43–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batinić-Haberle, I.; Spasojević, I.; Hambright, P.; Benov, L.; Crumbliss, A.L.; Fridovich, I. Relationship among Redox Potentials, Proton Dissociation Constants of Pyrrolic Nitrogens, and in Vivo and in Vitro Superoxide Dismutating Activities of Manganese(III) and Iron(III) Water-Soluble Porphyrins. Inorg. Chem. 1999, 38, 4011–4022. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Roberts, E.R.; Vujaskovic, Z.; Leong, K.W.; Spasojevic, I. SOD therapeutics: Latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid. Redox Signal. 2014, 20, 2372–2415. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Spasojevic, I. 25 years of development of Mn porphyrins—From mimics of superoxide dismutase enzymes to thiol signaling to clinical trials: The story of our life in the USA. J. Porphyr. Phthalocyanines 2019, 23, 1326–1335. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Carroll, D.W.; You, Y.; Chaiswing, L.; Wen, R.; Batinic-Haberle, I.; Bondada, S.; Liang, Y.; St Clair, D.K. A novel redox regulator, MnTnBuOE-2-PyP5+, enhances normal hematopoietic stem/progenitor cell function. Redox Biol. 2017, 12, 129–138. [Google Scholar] [CrossRef]
- Sheng, H.; Spasojevic, I.; Tse, H.M.; Jung, J.Y.; Hong, J.; Zhang, Z.; Piganelli, J.D.; Batinic-Haberle, I.; Warner, D.S. Neuroprotective Efficacy from a Lipophilic Redox-Modulating Mn(III) N-Hexylpyridylporphyrin, MnTnHex-2-PyP: Rodent Models of Ischemic Stroke and Subarachnoid Hemorrhage. J. Pharmacol. Exp. Ther. 2011, 338, 906–916. [Google Scholar] [CrossRef] [Green Version]
- Leu, D.; Spasojevic, I.; Nguyen, H.; Deng, B.; Tovmasyan, A.; Weitner, T.; Sampaio, R.S.; Batinic-Haberle, I.; Huang, T.T. CNS bioavailability and radiation protection of normal hippocampal neurogenesis by a lipophilic Mn porphyrin-based superoxide dismutase mimic, MnTnBuOE-2-PyP5. Redox Biol. 2017, 12, 864–871. [Google Scholar] [CrossRef]
- Spasojevic, I.; Miriyala, S.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; Batinic-Haberle, I.; St. Clair, D. Lipophilicity of Mn(III) N-alkylpyridylporphyrins dominates their accumulation within mitochondria and therefore in vivo efficacy. A mouse study. Free Radic. Biol. Med. 2011, 51, S98. [Google Scholar] [CrossRef]
- Cline, J.M.; Dugan, G.; Bourland, J.D.; Perry, D.L.; Stitzel, J.D.; Weaver, A.A.; Jiang, C.; Tovmasyan, A.; Owzar, K.; Spasojevic, I.; et al. Post-Irradiation Treatment with a Superoxide Dismutase Mimic, MnTnHex-2-PyP(5+), Mitigates Radiation Injury in the Lungs of Non-Human Primates after Whole-Thorax Exposure to Ionizing Radiation. Antioxidants (Basel) 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovmasyan, A.; Weitner, T.; Sheng, H.; Lu, M.; Rajic, Z.; Warner, D.S.; Spasojevic, I.; Reboucas, J.S.; Benov, L.; Batinic-Haberle, I. Differential Coordination Demands in Fe versus Mn Water-Soluble Cationic Metalloporphyrins Translate into Remarkably Different Aqueous Redox Chemistry and Biology. Inorg. Chem. 2013, 52, 5677–5691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, H.; Chaparro, R.E.; Sasaki, T.; Izutsu, M.; Pearlstein, R.D.; Tovmasyan, A.; Warner, D.S. Metalloporphyrins as therapeutic catalytic oxidoreductants in central nervous system disorders. Antioxid. Redox Signal. 2014, 20, 2437–2464. [Google Scholar] [CrossRef] [PubMed]
- Weitner, T.; Kos, I.; Sheng, H.; Tovmasyan, A.; Reboucas, J.S.; Fan, P.; Warner, D.S.; Vujaskovic, Z.; Batinic-Haberle, I.; Spasojevic, I. Comprehensive pharmacokinetic studies and oral bioavailability of two Mn porphyrin-based SOD mimics, MnTE-2-PyP(5+) and MnTnHex-2-PyP(5+). Free Radic. Biol. Med. 2013, 58, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Rajic, Z.; Tovmasyan, A.; Spasojevic, I.; Sheng, H.; Lu, M.; Li, A.M.; Gralla, E.B.; Warner, D.S.; Benov, L.; Batinic-Haberle, I. A new SOD mimic, Mn(III) ortho N-butoxyethylpyridylporphyrin, combines superb potency and lipophilicity with low toxicity. Free Radic. Biol. Med. 2012, 52, 1828–1834. [Google Scholar] [CrossRef] [Green Version]
- Spasojevic, I.; Menzeleev, R.; White, P.S.; Fridovich, I. Rotational isomers of N-alkylpyridylporphyrins and their metal complexes. HPLC separation, H-1 NMR and X-ray structural characterization, electrochemistry, and catalysis of O-2(center dot-) disproportionation. Inorg. Chem. 2002, 41, 5874–5881. [Google Scholar] [CrossRef]
- Sheng, H.; Enghild, J.J.; Bowler, R.; Patel, M.; Batinic-Haberle, I.; Calvi, C.L.; Day, B.J.; Pearlstein, R.D.; Crapo, J.D.; Warner, D.S. Effects of metalloporphyrin catalytic antioxidants in experimental brain ischemia. Free Radic. Biol. Med. 2002, 33, 947–961. [Google Scholar] [CrossRef]
- Sheng, H.; Yang, W.; Fukuda, S.; Tse, H.M.; Paschen, W.; Johnson, K.; Batinic-Haberle, I.; Crapo, J.D.; Pearlstein, R.D.; Piganelli, J.; et al. Long-term neuroprotection from a potent redox-modulating metalloporphyrin in the rat. Free Radic. Biol. Med. 2009, 47, 917–923. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Yu, S.; Yu, Z.; Sheng, H.; Li, Y.; Liu, S.; Warner, D.S.; Paschen, W.; Yang, W. XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation Is Neuroprotective in Ischemic Stroke in Young Mice and Its Impairment in Aged Mice Is Rescued by Thiamet-G. Stroke 2017, 48, 1646–1654. [Google Scholar] [CrossRef]
- Spasojevic, I.; Chen, Y.; Noel, T.J.; Fan, P.; Zhang, L.; Reboucas, J.S.; St Clair, D.K.; Batinic-Haberle, I. Pharmacokinetics of the potent redox-modulating manganese porphyrin, MnTE-2-PyP(5+), in plasma and major organs of B6C3F1 mice. Free Radic. Biol. Med. 2008, 45, 943–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.B.; Hunt, J.A.; Groves, J.T. Mechanisms of iron porphyrin reactions with peroxynitrite. J. Am. Chem. Soc. 1998, 120, 7493–7501. [Google Scholar] [CrossRef]
- Stern, M.K.; Jensen, M.P.; Kramer, K. Peroxynitrite Decomposition Catalysts. J. Am. Chem. Soc. 1996, 118, 8735–8736. [Google Scholar] [CrossRef]
- Chen, H.S.; Chen, X.M.; Feng, J.H.; Liu, K.J.; Qi, S.H.; Shen, J.G. Peroxynitrite Decomposition Catalyst Reduces Delayed Thrombolysis-induced Hemorrhagic Transformation in Ischemia-reperfused Rat Brains. CNS Neurosci. Ther. 2015, 21, 585–590. [Google Scholar] [CrossRef]
- Salvemini, D.; Wang, Z.Q.; Stern, M.K.; Currie, M.G.; Misko, T.P. Peroxynitrite decomposition catalysts: Therapeutics for peroxynitrite-mediated pathology. Proc. Natl. Acad. Sci. USA 1998, 95, 2659–2663. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Wang, K.; Shang, J.; Cao, C.; Zhen, P.; Liu, X.; Wang, W.; Zhang, H.; Du, Y.; Liu, H. Anti-peroxynitrite treatment ameliorated vasorelaxation of resistance arteries in aging rats: Involvement with NO-sGC-cGKs pathway. PLoS ONE 2014, 9, e104788. [Google Scholar] [CrossRef]
- Jangra, A.; Datusalia, A.K.; Sharma, S.S. Reversal of neurobehavioral and neurochemical alterations in STZ-induced diabetic rats by FeTMPyP, a peroxynitrite decomposition catalyst and 1,5-Isoquinolinediol a poly(ADP-ribose) polymerase inhibitor. Neurol. Res. 2014, 36, 619–626. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Gu, Y.; Peng, T.; Yang, D.; Chang, R.C.; So, K.F.; Liu, K.; Shen, J. Baicalin can scavenge peroxynitrite and ameliorate endogenous peroxynitrite-mediated neurotoxicity in cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 2013, 150, 116–124. [Google Scholar] [CrossRef]
- Palomares, S.M.; Gardner-Morse, I.; Sweet, J.G.; Cipolla, M.J. Peroxynitrite decomposition with FeTMPyP improves plasma-induced vascular dysfunction and infarction during mild but not severe hyperglycemic stroke. J. Cereb. Blood Flow Metab. 2012, 32, 1035–1045. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dhar, A.; Kaundal, R.K. FeTPPS protects against global cerebral ischemic-reperfusion injury in gerbils. Pharmacol. Res. 2007, 55, 335–342. [Google Scholar] [CrossRef]
- Slosky, L.M.; Vanderah, T.W. Therapeutic potential of peroxynitrite decomposition catalysts: A patent review. Expert Opin. Ther. Pat. 2015, 25, 443–466. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanisms and Process in Physiological Evolution; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Tunnicliff, G. Pharmacology and function of imidazole 4-acetic acid in brain. Gen. Pharmacol. 1998, 31, 503–509. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Dominguez, M.L.; Sá-Almeida, A.M. Excitatory Action of Imidazole on Evoked Transmitter Release from the Phrenic Nerve and on Potassium-stimulated 45Ca Uptake by Synaptosomes in the Rat. In Aminopyridines and Similarly Acting Drugs: Effects on Nerves, Muscles and Synapses; Lechat, P., Thesleff, S., Bowman, W.C., Eds.; Pergamon Press: Oxford, UK, 1982; p. 227. [Google Scholar]
- Liu, T.; Ji, R.R. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci. Bull. 2012, 28, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, N.; Malkova, E.; Syrbu, S.; Gubarev, Y.; Nikitin, D. Investigation of Interactions Between Cationic and Anionic Porphyrins and BSA in Aqueous Media. Int. J. Biochem. Biophysics 2013, 2, 13–18. [Google Scholar]
- Jiménez, H.R.; Arbona, M. Spectroscopic studies of water-soluble superstructured iron(III) porphyrin. Interaction with the bovine serum albumin protein. J. Coord. Chem. 2018, 71, 890–905. [Google Scholar] [CrossRef]
- Chatterjee, S.; Srivastava, T.S. Spectral investigations of the interaction of some porphyrins with bovine serum albumin. J. Porphyr. Phthalocyanines 2000, 4, 147–157. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Gibbs, E.J.; Gaudemer, A.; Antebi, A.; Bassner, S.; De Poy, L.; Turner, D.H.; Williams, A.; Laplace, F.; Lansard, M.H.; et al. Molecular complexes of nucleosides and nucleotides with a monomeric cationic porphyrin and some of its metal derivatives. J. Am. Chem. Soc. 1985, 107, 8179–8186. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.F.; Gibbs, E.J.; Villafranca, J.J. Interactions of porphyrins with nucleic acids. Biochemistry 1983, 22, 2406–2414. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Tovmasyan, A.; Sheng, H.; Xu, B.; Sampaio, R.S.; Reboucas, J.S.; Warner, D.S.; Batinic-Haberle, I.; Spasojevic, I. Fe Porphyrin-Based SOD Mimic and Redox-Active Compound, (OH)FeTnHex-2-PyP4+, in a Rodent Ischemic Stroke (MCAO) Model: Efficacy and Pharmacokinetics as Compared to Its Mn Analogue, (H2O)MnTnHex-2-PyP5+. Antioxidants 2020, 9, 467. https://doi.org/10.3390/antiox9060467
Li L, Tovmasyan A, Sheng H, Xu B, Sampaio RS, Reboucas JS, Warner DS, Batinic-Haberle I, Spasojevic I. Fe Porphyrin-Based SOD Mimic and Redox-Active Compound, (OH)FeTnHex-2-PyP4+, in a Rodent Ischemic Stroke (MCAO) Model: Efficacy and Pharmacokinetics as Compared to Its Mn Analogue, (H2O)MnTnHex-2-PyP5+. Antioxidants. 2020; 9(6):467. https://doi.org/10.3390/antiox9060467
Chicago/Turabian StyleLi, Litao, Artak Tovmasyan, Huaxin Sheng, Bin Xu, Romulo S. Sampaio, Julio S. Reboucas, David S. Warner, Ines Batinic-Haberle, and Ivan Spasojevic. 2020. "Fe Porphyrin-Based SOD Mimic and Redox-Active Compound, (OH)FeTnHex-2-PyP4+, in a Rodent Ischemic Stroke (MCAO) Model: Efficacy and Pharmacokinetics as Compared to Its Mn Analogue, (H2O)MnTnHex-2-PyP5+" Antioxidants 9, no. 6: 467. https://doi.org/10.3390/antiox9060467
APA StyleLi, L., Tovmasyan, A., Sheng, H., Xu, B., Sampaio, R. S., Reboucas, J. S., Warner, D. S., Batinic-Haberle, I., & Spasojevic, I. (2020). Fe Porphyrin-Based SOD Mimic and Redox-Active Compound, (OH)FeTnHex-2-PyP4+, in a Rodent Ischemic Stroke (MCAO) Model: Efficacy and Pharmacokinetics as Compared to Its Mn Analogue, (H2O)MnTnHex-2-PyP5+. Antioxidants, 9(6), 467. https://doi.org/10.3390/antiox9060467