Polymyxins–Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment
Abstract
:1. Introduction
2. Curcumin
2.1. Antibacterial Activity of Curcumin
2.2. Effect of Curcumin on Bacteria or Its Toxin-Induced Inflammatory Response
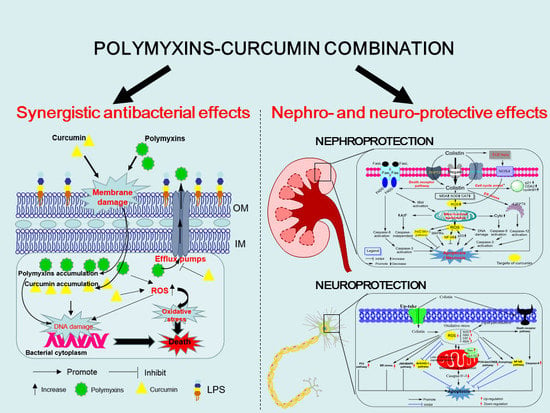
3. Synergistic Antibacterial Effects of the Polymyxin in Combination with Curcumin
4. Polymyxin-Induced Nephrotoxicity and Protective Effect of Curcumin
5. Colistin-Induced Neurotoxicity and Protective Effects of Curcumin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Koyama, Y.; Kurosasa, A.; Tsuchiya, A.; Takakuta, K. A new antibiotic “colistin” produced by spore-forming soil bacteria. J. Antibiot. 1950, 3, 457–458. [Google Scholar]
- Braine, T. Race against time to develop new antibiotics. Bull. World Health Organ. 2011, 89, 88–89. [Google Scholar]
- Zowawi, H.M.; Harris, P.N.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The emerging threat of multidrug-resistant gram-negative bacteria in urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar] [CrossRef]
- Demiraslan, H.; Cevahir, F.; Berk, E.; Metan, G.; Cetin, M.; Alp, E. Is surveillance for colonization of carbapenem-resistant gram-negative bacteria important in adult bone marrow transplantation units? Am. J. Infect. Control 2017, 45, 735–739. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lob, S.H.; Kazmierczak, K.M.; Hawser, S.P.; Magnet, S.; Young, K.; Motyl, M.R.; Sahm, D.F. In vitro activity of imipenem/relebactam against gram-negative eskape pathogens isolated in 17 european countries: 2015 smart surveillance programme. J. Antimicrob. Chemother. 2018, 73, 1872–1879. [Google Scholar] [CrossRef]
- Adams-Sapper, S.; Gayoso, A.; Riley, L.W. Stress-adaptive responses associated with high-level carbapenem resistance in kpc-producing klebsiella pneumoniae. J. Pathog. 2018, 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant gram-negative bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar]
- Oliveira, V.D.; Rubio, F.G.; Almeida, M.T.; Nogueira, M.C.; Pignatari, A.C. Trends of 9416 multidrug-resistant gram-negative bacteria. Rev. Assoc. Med. Bras. 2015, 61, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014, 59, S335–S339. [Google Scholar] [CrossRef]
- Nation, R.L.; Velkov, T.; Li, J. Colistin and polymyxin b: Peas in a pod, or chalk and cheese? Clin. Infect. Dis. 2014, 59, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Nation, R.L.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Forrest, A.; Paterson, D.L.; Li, J.; Silveira, F.P. Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 2017, 64, 565–571. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in china: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Napier, B.A.; Band, V.; Burd, E.M.; Weiss, D.S. Colistin heteroresistance in enterobacter cloacae is associated with cross-resistance to the host antimicrobial lysozyme. Antimicrob. Agents Chemother. 2014, 58, 5594–5597. [Google Scholar] [CrossRef] [Green Version]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef] [Green Version]
- Hartzell, J.D.; Neff, R.; Ake, J.; Howard, R.; Olson, S.; Paolino, K.; Vishnepolsky, M.; Weintrob, A.; Wortmann, G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin. Infect. Dis. 2009, 48, 1724–1728. [Google Scholar] [CrossRef]
- Ni, W.T.; Shao, X.D.; Di, X.Z.; Cui, J.C.; Wang, R.; Liu, Y.N. In vitro synergy of polymyxins with other antibiotics for acinetobacter Baumannii: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2015, 45, 8–18. [Google Scholar] [CrossRef]
- O’Hara, J.A.; Ambe, L.A.; Casella, L.G.; Townsend, B.M.; Pelletier, M.R.; Ernst, R.K.; Shanks, R.M.Q.; Doi, Y. Activities of vancomycin-containing regimens against colistin-resistant acinetobacter Baumannii clinical strains. Antimicrob. Agents Chemother. 2013, 57, 2103–2108. [Google Scholar] [CrossRef] [Green Version]
- Salam, A.M.; Quave, C.L. Opportunities for plant natural products in infection control. Curr. Opin. Microbiol. 2018, 45, 189–194. [Google Scholar] [CrossRef]
- Silver, L.L. Natural products as a source of drug leads to overcome drug resistance. Future Microbiol. 2015, 10, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.W.; Sharili, A.S.; La Ragione, R.M.; Wareham, D.W. In vitro antibacterial activity of curcumin-polymyxin b combinations against multidrug-resistant bacteria associated with traumatic wound infections. J. Nat. Prod. 2016, 79, 1702–1706. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, P.; Capalash, N. Curcumin alleviates persistence of acinetobacter Baumannii against colistin. Sci. Rep. 2018, 8, 11029. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Zhang, Y.; Xiang, B.; Hoyer, D.; Shen, J.; Velkov, T.; Tang, S. Curcumin attenuates colistin-induced peripheral neurotoxicity in mice. ACS Infect. Dis. 2020, 6, 715–724. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Li, D.; Xie, S.; Xiao, X.; Velkov, T. Curcumin attenuates colistin-induced neurotoxicity in n2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018, 55, 421–434. [Google Scholar] [CrossRef]
- Edrees, N.E.; Galal, A.A.A.; Monaem, A.R.A.; Beheiry, R.R.; Metwally, M.M.M. Curcumin alleviates colistin-induced nephrotoxicity and neurotoxicity in rats via attenuation of oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2018, 294, 56–64. [Google Scholar] [CrossRef]
- Lin, X.P.; Xue, C.; Zhang, J.M.; Wu, W.J.; Chen, X.Y.; Zeng, Y.M. Curcumin inhibits lipopolysaccharide-induced mucin 5ac hypersecretion and airway inflammation via nuclear factor erythroid 2-related factor 2. Chin. Med. J. 2018, 131, 1686–1693. [Google Scholar] [CrossRef]
- Lobo de Sa, F.D.; Butkevych, E.; Nattramilarasu, P.K.; Fromm, A.; Mousavi, S.; Moos, V.; Golz, J.C.; Stingl, K.; Kittler, S.; Seinige, D.; et al. Curcumin mitigates immune-induced epithelial barrier dysfunction by campylobacter Jejuni. Int. J. Mol. Sci. 2019, 20, 4830. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.M.; Lopes, T.; Oleastro, M.; Gato, I.V.; Floch, P.; Benejat, L.; Chaves, P.; Pereira, T.; Seixas, E.; Machado, J.; et al. Curcumin inhibits gastric inflammation induced by helicobacter pylori infection in a mouse model. Nutrients 2015, 7, 306–320. [Google Scholar] [CrossRef] [Green Version]
- Schaefers, M.M.; Breshears, L.M.; Anderson, M.J.; Lin, Y.C.; Grill, A.E.; Panyam, J.; Southern, P.J.; Schlievert, P.M.; Peterson, M.L. Epithelial proinflammatory response and curcumin-mediated protection from Staphylococcal toxic shock syndrome toxin-1. PLoS ONE 2012, 7, e32813. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Miao, H.; Li, X.; Hu, Y.; Sun, H.; Hou, Y. Curcumin inhibits placental inflammation to ameliorate lps-induced adverse pregnancy outcomes in mice via upregulation of phosphorylated akt. Inflamm. Res. 2017, 66, 177–185. [Google Scholar] [CrossRef]
- Kumari, A.; Dash, D.; Singh, R. Curcumin inhibits Lipopolysaccharide (lps)-induced endotoxemia and airway inflammation through modulation of sequential release of inflammatory mediators (tnf-alpha and tgf-beta1) in murine model. Inflammopharmacology 2017, 25, 329–341. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Lao, C.D.; Ruffin, M.T.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Malo, D.; Villaron-Casares, C.A.; Alarcon-Jimenez, J.; Miranda, M.; Diaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a therapeutic option in retinal diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation; diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: New York, NY, USA, 2007; Volume 595. [Google Scholar]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.H.; Du, Z.Y. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef]
- Poonam, T.; Madhuri, S.; Himani, K.; Anita, K.; Kasturi, M.; One, Z.D.J.P. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar]
- Praditya, D.; Kirchhoff, L.; Bruning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin inhibits ftsz assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Van Acker, H.; Coenye, T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017, 25, 456–466. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Li, Z.; Zhang, X.; Lu, N.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q. Curcumin-mediated photodynamic inactivation (pdi) against dh5alpha contaminated in oysters and cellular toxicological evaluation of pdi-treated oysters. Photodiagn. Photodyn. 2019, 26, 244–251. [Google Scholar] [CrossRef]
- Freitas, M.A.; Pereira, A.H.; Pinto, J.G.; Casas, A.; Ferreira-Strixino, J. Bacterial viability after antimicrobial photodynamic therapy with curcumin on multiresistant staphylococcus aureus. Future Microbiol. 2019, 14, 739–748. [Google Scholar] [CrossRef]
- Rudrappa, T.; Bais, H.P. Curcumin, a known phenolic from curcuma longa, attenuates the virulence of pseudomonas aeruginosa pao1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niamsa, N.; Sittiwet, C. Antimicrobial activity of curcuma longa aqueous extract. J. Pharmacol. Toxicol. 2009, 4, 174–177. [Google Scholar]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.F.; Wang, J.N.; Li, Z.; Wei, B.; Jin, J.; Gao, L.; Li, H.D.; Li, J.; Chen, H.Y.; Meng, X.M. 7-hydroxycoumarin protects against cisplatin-induced acute kidney injury by inhibiting necroptosis and promoting sox9-mediated tubular epithelial cell proliferation. Phytomedicine 2020, 69, 153202. [Google Scholar] [CrossRef]
- Shlar, I.; Droby, S.; Rodov, V. Modes of antibacterial action of curcumin under dark and light conditions: A toxicoproteomics approach. J. Proteom. 2017, 160, 8–20. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Diao, R.; Liu, J.; Kang, Y.; Wang, X.; Shi, L. Curcumin attenuates Staphylococcus aureus-induced acute lung injury. Clin. Respir. J. 2015, 9, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits lps-induced neuroinflammation by promoting microglial m2 polarization via trem2/ tlr4/ nf-kappab pathways in bv2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Bai, X.; Oberley-Deegan, R.E.; Bai, A.; Ovrutsky, A.R.; Kinney, W.H.; Weaver, M.; Zhang, G.; Honda, J.R.; Chan, E.D. Curcumin enhances human macrophage control of mycobacterium tuberculosis infection. Respirology 2016, 21, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Foryst-Ludwig, A.; Neumann, M.; Schneider-Brachert, W.; Naumann, M. Curcumin blocks nf-kappab and the motogenic response in helicobacter pylori-infected epithelial cells. Biochem. Biophys. Res. Commun. 2004, 316, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; De, R.; Pal, I.; Mukhopadhyay, A.K.; Saha, D.R.; Swarnakar, S. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS ONE 2011, 6, e16306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.P.; Guo, Q.; Li, X.; Tang, T.T.; Li, C.L.; Wang, H.X.; Sun, Y.X.; Feng, Q.; Ma, C.H.; Gao, C.J.; et al. Curcumin suppresses il-1 beta secretion and prevents inflammation through inhibition of the nlrp3 inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.C.; Lee, W.; Bae, J.S. Vascular anti-inflammatory effects of curcumin on hmgb1-mediated responses in vitro. Inflamm. Res. 2011, 60, 1161–1168. [Google Scholar] [CrossRef]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflamm. 2011, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [Green Version]
- Sampson, T.R.; Liu, X.; Schroeder, M.R.; Kraft, C.S.; Burd, E.M.; Weiss, D.S. Rapid killing of acinetobacter Baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob. Agents Chemother. 2012, 56, 5642–5649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Qin, W.; Lin, J.; Fang, S.; Qiu, J. Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed. Res. Int. 2015, 2015, 679109. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.D.; Trent, M.S. Fortifying the barrier: The impact of lipid a remodelling on bacterial pathogenesis. Nat. Rev. Microbiol. 2013, 11, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofteridis, D.P.; Alexopoulou, C.; Valachis, A.; Maraki, S.; Dimopoulou, D.; Georgopoulos, D.; Samonis, G. Aerosolized plus intravenous colistin versus intravenous colistin alone for the treatment of ventilator-associated pneumonia: A matched case-control study. Clin. Infect. Dis. 2010, 51, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.B.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular mechanisms of colistin-induced nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef] [Green Version]
- Dezoti-Fonseca, C.; Watanabe, M.; Vattimo-Mde, F. Role of heme oxygenase-1 in polymyxin b-induced nephrotoxicity in rats. Antimicrob. Agents Chemother. 2012, 56, 5082–5087. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Tang, S.; Wang, Y.; Velkov, T.; Xiao, X. Baicalein acts as a nephroprotectant that ameliorates colistin-induced nephrotoxicity by activating the antioxidant defence mechanism of the kidneys and down-regulating the inflammatory response. J. Antimicrob. Chemother. 2017, 72, 2562–2569. [Google Scholar] [CrossRef] [Green Version]
- Wallace, S.J.; Li, J.; Nation, R.L.; Rayner, C.R.; Taylor, D.; Middleton, D.; Milne, R.W.; Coulthard, K.; Turnidge, J.D. Subacute toxicity of colistin methanesulfonate in rats: Comparison of various intravenous dosage regimens. Antimicrob. Agents Chemother. 2008, 52, 1159–1161. [Google Scholar] [CrossRef] [Green Version]
- Ghlissi, Z.; Hakim, A.; Mnif, H.; Ayadi, F.M.; Zeghal, K.; Rebai, T.; Sahnoun, Z. Evaluation of colistin nephrotoxicity administered at different doses in the rat model. Ren. Fail. 2013, 35, 1130–1135. [Google Scholar] [CrossRef] [Green Version]
- Keirstead, N.D.; Wagoner, M.P.; Bentley, P.; Blais, M.; Brown, C.; Cheatham, L.; Ciaccio, P.; Dragan, Y.; Ferguson, D.; Fikes, J.; et al. Early prediction of polymyxin-induced nephrotoxicity with next-generation urinary kidney injury biomarkers. Toxicol. Sci. 2014, 137, 278–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, M.A.K.; Roberts, K.D.; Yu, H.D.H.; Liu, B.Y.; Schofield, A.V.; James, S.A.; Howard, D.L.; Nation, R.L.; Rogers, K.; de Jonge, M.D.; et al. Significant accumulation of polymyxin in single renal tubular cells: A medicinal chemistry and triple correlative microscopy approach. Anal. Chem. 2015, 87, 1590–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, B.; Azad, M.A.K.; Wang, J.; Nation, R.L.; Thompson, P.E.; Roberts, K.D.; Velkov, T.; Li, J. Imaging the distribution of polymyxins in the kidney. J. Antimicrob. Chemother. 2015, 70, 827–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velkov, T.; Yun, B.; Schneider, E.K.; Azad, M.A.; Dolezal, O.; Morris, F.C.; Nation, R.L.; Wang, J.; Chen, K.; Yu, H.H.; et al. A novel chemical biology approach for mapping of polymyxin lipopeptide antibody binding epitopes. ACS Infect. Dis. 2016, 13, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Manchandani, P.; Zhou, J.; Ledesma, K.R.; Truong, L.D.; Chow, D.S.; Eriksen, J.L.; Tam, V.H. Characterization of polymyxin b biodistribution and disposition in an animal model. Antimicrob. Agents Chemother. 2016, 60, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Sivanesan, S.S.; Azad, M.A.K.; Schneider, E.K.; Ahmed, M.U.; Huang, J.; Wang, J.; Li, J.; Nation, R.L.; Velkov, T. Gelofusine ameliorates colistin-induced nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e00985-17. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.A.K.; Sivanesan, S.; Wang, J.; Chen, K.; Nation, R.L.; Thompson, P.E.; Roberts, K.D.; Velkov, T.; Li, J. Methionine ameliorates polymyxin-induced nephrotoxicity by attenuating cellular oxidative stress. Antimicrob. Agents Chemother. 2017, 62, e01254-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, K.D.; Azad, M.A.; Wang, J.; Horne, A.S.; Thompson, P.E.; Nation, R.L.; Velkov, T.; Li, J. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin b and colistin: Last-line antibiotics against multidrug-resistant gram-negative bacteria. ACS Infect. Dis. 2015, 1, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Chan, T.; Xu, C.; Zhu, L.; Zhou, Q.T.; Roberts, K.D.; Chan, H.K.; Li, J.; Zhou, F. Human oligopeptide transporter 2 (pept2) mediates cellular uptake of polymyxins. J. Antimicrob. Chemother. 2016, 71, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Tang, S.; Deng, S.; Zhang, S.; Zhou, Y.; Velkov, T.; Li, J.; Xiao, X. Lycopene attenuates colistin-induced nephrotoxicity in mice via activation of the nrf2/ho-1 pathway. Antimicrob. Agents Chemother. 2015, 59, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Li, J.; Tang, S.; Li, J.; Xiao, X. Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother. 2014, 58, 4075–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousef, J.M.; Chen, G.; Hill, P.A.; Nation, R.L.; Li, J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J. Antimicrob. Chemother. 2012, 67, 452–459. [Google Scholar] [CrossRef]
- Yousef, J.M.; Chen, G.; Hill, P.A.; Nation, R.L.; Li, J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob. Agents Chemother. 2011, 55, 4044–4049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Wang, J.; Nation, R.L.; Li, J.; Turnidge, J.D.; Coulthard, K.; Milne, R.W. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob. Agents Chemother. 2009, 53, 2857–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Yamaguchi, H.; Ogura, J.; Kobayashi, M.; Yamada, T.; Iseki, K. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob. Agents Chemother. 2013, 57, 6319–6324. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Aoki, N.; Kuwahara, S.; Hosojima, M.; Kaseda, R.; Goto, S.; Iida, T.; De, S.; Kabasawa, H.; Kaneko, R.; et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. JASN 2017, 28, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Visentin, M.; Gai, Z.; Torozi, A.; Hiller, C.; Kullak-Ublick, G.A. Colistin is substrate of the carnitine/organic cation transporter 2 (octn2, slc22a5). Drug Metab. Dispos. 2017, 45, 1240–1244. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.D.; Luo, J.; Jia, L.H.; Wang, X.Y.; Xun, Z.K.; Liu, M. Cytochrome c suppresses renal accumulation and nephrotoxicity of polymyxin b. Hum. Exp. Toxicol. 2019, 38, 193–200. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Akter, J.; Rogers, K.L.; Nation, R.L.; Velkov, T.; Li, J. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob. Agents Chemother. 2015, 59, 2136–2143. [Google Scholar] [CrossRef] [Green Version]
- Eadon, M.T.; Hack, B.K.; Alexander, J.J.; Xu, C.; Dolan, M.E.; Cunningham, P.N. Cell cycle arrest in a model of colistin nephrotoxicity. Physiol. Genom. 2013, 45, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Yun, B.; Zhang, T.; Azad, M.A.K.; Wang, J.; Nowell, C.J.; Kalitsis, P.; Velkov, T.; Hudson, D.F.; Li, J. Polymyxin b causes DNA damage in hk-2 cells and mice. Arch. Toxicol. 2018, 92, 2259–2271. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Bae, E.; Kim, J.H.; Jang, H.N.; Cho, H.S.; Chang, S.H.; Park, D.J. The aqueous extract of aged black garlic ameliorates colistin-induced acute kidney injury in rats. Ren. Fail. 2019, 41, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, B.Y.; Park, S.R.; Cho, S.; Yu, S.L.; Lee, H.Y.; Park, C.G.; Kang, J.; Jung, D.Y.; Park, M.H.; Hwang, W.M.; et al. Tgf-beta-mediated nadph oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. J. Antimicrob. Chemother. 2018, 73, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pan, X.; Fu, H.; Zheng, Y.; Dai, Y.; Yin, Y.; Chen, Q.; Hao, Q.; Bao, D.; Hou, D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 2017, 7, 10114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Peng, X.; Zhu, J.; Liu, G.; Chen, X.; Tang, C.; Liu, H.; Liu, F.; Peng, Y. Protective effects of curcumin on acute gentamicin-induced nephrotoxicity in rats. Can. J. Physiol. Pharm. 2015, 93, 275–282. [Google Scholar] [CrossRef]
- Deck, L.M.; Hunsaker, L.A.; Vander Jagt, T.A.; Whalen, L.J.; Royer, R.E.; Vander Jagt, D.L. Activation of antioxidant nrf2 signaling by enone analogues of curcumin. Eur. J. Med. Chem. 2018, 143, 854–865. [Google Scholar] [CrossRef]
- Dai, C.; Li, B.; Zhou, Y.; Li, D.; Zhang, S.; Li, H.; Xiao, X.; Tang, S. Curcumin attenuates quinocetone induced apoptosis and inflammation via the opposite modulation of nrf2/ho-1 and nf-kb pathway in human hepatocyte l02 cells. Food Chem. Toxicol. 2016, 95, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Dominguez, B.; Aparicio-Trejo, O.E.; Garcia-Arroyo, F.E.; Leon-Contreras, J.C.; Tapia, E.; Molina-Jijon, E.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017, 107, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rojas, S.H.; Tapia, E.; Briones-Herrera, A.; Aparicio-Trejo, O.E.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Pedraza-Chaverri, J. Curcumin prevents potassium dichromate (k2cr2o7)-induced renal hypoxia. Food Chem. Toxicol. 2018, 121, 472–482. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Garcia-Caballero, C.; Palomino-Antolin, A.; Rubio-Navarro, A.; Vazquez-Carballo, C.; Herencia, C.; Martin-Sanchez, D.; Farre-Alins, V.; Egea, J.; Cannata, P.; et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019, 33, 8961–8975. [Google Scholar] [CrossRef]
- Molina-Jijon, E.; Aparicio-Trejo, O.E.; Rodriguez-Munoz, R.; Leon-Contreras, J.C.; Del Carmen Cardenas-Aguayo, M.; Medina-Campos, O.N.; Tapia, E.; Sanchez-Lozada, L.G.; Hernandez-Pando, R.; Reyes, J.L.; et al. The nephroprotection exerted by curcumin in maleate-induced renal damage is associated with decreased mitochondrial fission and autophagy. Biofactors 2016, 42, 686–702. [Google Scholar] [CrossRef]
- Sankar, P.; Telang, A.G.; Kalaivanan, R.; Karunakaran, V.; Suresh, S.; Kesavan, M. Oral nanoparticulate curcumin combating arsenic-induced oxidative damage in kidney and brain of rats. Toxicol. Ind. Health 2016, 32, 410–421. [Google Scholar] [CrossRef]
- Benzer, F.; Kandemir, F.M.; Kucukler, S.; Comakli, S.; Caglayan, C. Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in wistar rats: By modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch. Physiol. Biochem. 2018, 124, 448–457. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit. Care 2006, 10, R27. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, L.; Doan, T.L.; Smith, M.A. Neurotoxicity in patients treated with intravenous polymyxin b: Two case reports. Am. J. Health Syst. Pharm. 2009, 66, 345–347. [Google Scholar] [CrossRef]
- Wahby, K.; Chopra, T.; Chandrasekar, P. Intravenous and inhalational colistin-induced respiratory failure. Clin. Infect. Dis. 2010, 50, e38–e40. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Tang, S.; Li, J.; Wang, J.; Xiao, X. Effects of colistin on the sensory nerve conduction velocity and f-wave in mice. Basic Clin. Pharmacol. Toxicol. 2014, 115, 577–580. [Google Scholar] [CrossRef] [Green Version]
- Bosso, J.A.; Liptak, C.A.; Seilheimer, D.K.; Harrison, G.M. Toxicity of colistin in cystic fibrosis patients. DICP 1991, 25, 1168–1170. [Google Scholar] [CrossRef]
- John, J.F.; Falci, D.R.; Rigatto, M.H.; Oliveira, R.D.; Kremer, T.G.; Zavascki, A.P. Severe infusion-related adverse events and renal failure in patients receiving high-dose intravenous polymyxin b. Antimicrob. Agents Chemother. 2018, 62, e01617-17. [Google Scholar] [CrossRef] [Green Version]
- Hitchcock, S.A. Blood-brain barrier permeability considerations for cns-targeted compound library design. Curr. Opin. Chem. Biol. 2008, 12, 318–323. [Google Scholar] [CrossRef]
- Jin, L.; Nation, R.L.; Li, J.; Nicolazzo, J.A. Species-dependent blood-brain barrier disruption of lipopolysaccharide: Amelioration by colistin in vitro and in vivo. Antimicrob. Agents Chemother. 2013, 57, 4336–4342. [Google Scholar] [CrossRef] [Green Version]
- Loho, T.; Dharmayanti, A. Colistin: An antibiotic and its role in multiresistant gram-negative infections. Acta Med. Indones. 2015, 47, 157–168. [Google Scholar]
- Jin, L.; Li, J.; Nation, R.L.; Nicolazzo, J.A. Effect of systemic infection induced by pseudomonas aeruginosa on the brain uptake of colistin in mice. Antimicrob. Agents Chemother. 2012, 56, 5240–5246. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Li, J.; Nation, R.L.; Nicolazzo, J.A. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob. Agents Chemother. 2011, 55, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Li, J.; Nation, R.L.; Nicolazzo, J.A. Brain penetration of colistin in mice assessed by a novel high-performance liquid chromatographic technique. Antimicrob. Agents Chemother. 2009, 53, 4247–4251. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yi, M.; Chen, X.; Muhammad, I.; Liu, F.; Li, R.; Li, J.; Li, J. Effects of colistin on amino acid neurotransmitters and blood-brain barrier in the mouse brain. Neurotoxicol. Teratol. 2016, 55, 32–37. [Google Scholar] [CrossRef]
- Dai, C.; Li, J.; Lin, W.; Li, G.; Sun, M.; Wang, F.; Li, J. Electrophysiology and ultrastructural changes in mouse sciatic nerve associated with colistin sulfate exposure. Toxicol. Mech. Methods 2012, 22, 592–596. [Google Scholar] [CrossRef]
- Dai, C.; Li, J.; Li, J. New insight in colistin induced neurotoxicity with the mitochondrial dysfunction in mice central nervous tissues. Exp. Toxicol. Pathol. 2013, 65, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Hoyer, D.; Schneider, E.K.; Velkov, T.; Xiao, X. Rapamycin confers neuroprotection against colistin-induced oxidative stress, mitochondria dysfunction, and apoptosis through the activation of autophagy and mtor/akt/creb signaling pathways. ACS Chem. Neurosci. 2018, 9, 824–837. [Google Scholar] [CrossRef]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Xiao, X.; Velkov, T. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction and oxidative stress. J. Antimicrob. Chemother. 2017, 72, 1635–1645. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, J.S.; Ravichandran, K.; Gil, H.W.; Song, H.Y.; Hong, S.Y. P-glycoprotein induction ameliorates colistin induced nephrotoxicity in cultured human proximal tubular cells. PLoS ONE 2015, 10, e0136075. [Google Scholar] [CrossRef] [Green Version]
- Daniel, H.; Kottra, G. The proton oligopeptide cotransporter family slc15 in physiology and pharmacology. Pflug. Arch. 2004, 447, 610–618. [Google Scholar]
- Spuch, C.; Ortolano, S.; Navarro, C. Lrp-1 and lrp-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer’s disease. Front. Physiol. 2012, 3, 269. [Google Scholar] [CrossRef] [Green Version]
- Courousse, T.; Bacq, A.; Belzung, C.; Guiard, B.; Balasse, L.; Louis, F.; Le Guisquet, A.M.; Gardier, A.M.; Schinkel, A.H.; Giros, B.; et al. Brain organic cation transporter 2 controls response and vulnerability to stress and gsk3beta signaling. Mol. Psychiatry 2015, 20, 889–900. [Google Scholar] [CrossRef]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tang, S.; Biao, X.; Xiao, X.; Chen, C.; Li, J. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol. Biol. Rep. 2019, 46, 1963–1972. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, D.; Li, J.; Li, J. Effect of colistin exposure on calcium homeostasis and mitochondria functions in chick cortex neurons. Toxicol. Mech. Methods 2013, 23, 281–288. [Google Scholar] [CrossRef]
- Stowe, D.F.; Camara, A.K. Mitochondrial reactive oxygen species production in excitable cells: Modulators of Mitochondrial and cell function. Antioxid. Redox Signal. 2009, 11, 1373–1414. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Tang, S.; Velkov, T.; Xiao, X. Colistin-induced apoptosis of neuroblastoma-2a cells involves the generation of reactive oxygen species, mitochondrial dysfunction, and autophagy. Mol. Neurobiol. 2016, 53, 4685–4700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Chen, C.; Wu, Z.; Miao, Y.; Muhammad, I.; Ding, L.; Tian, E.; Hu, W.; Ni, H.; Li, R.; et al. A dual role of p53 in regulating colistin-induced autophagy in pc-12 cells. Front. Pharm. 2017, 8, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.U.; Velkov, T.; Lin, Y.W.; Yun, B.; Nowell, C.J.; Zhou, F.; Zhou, Q.T.; Chan, K.; Azad, M.A.K.; Li, J. Potential toxicity of polymyxins in human lung epithelial cells. Antimicrob. Agents Chemother. 2017, 61, e02690-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Li, J.; Zhou, T.; Wang, C.; Zhang, H.; Wang, H. Colistin-induced apoptosis in pc12 cells: Involvement of the mitochondrial apoptotic and death receptor pathways. Int. J. Mol. Med. 2014, 33, 1298–1304. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Li, J.; Ciccotosto, G.D.; Cappai, R.; Tang, S.; Schneider-Futschik, E.K.; Hoyer, D.; Velkov, T.; Shen, J. Molecular mechanisms of neurotoxicity induced by polymyxins and chemoprevention. ACS Chem. Neurosci. 2019, 10, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Y.; Ding, W.; Jiang, G.; Lu, Z.; Li, L.; Wang, J.; Li, J.; Li, J. Autophagy regulates colistin-induced apoptosis in pc-12 cells. Antimicrob. Agents Chemother. 2015, 59, 2189–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Pan, X.; Li, T.; Zhang, C.; Shi, N. Lycium barbarum polysaccharides protect against trimethyltin chloride-induced apoptosis via sonic hedgehog and pi3k/akt signaling pathways in mouse neuro-2a cells. Oxid. Med. Cell. Longev. 2016, 2016, 9826726. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Miao, Y.; Muhammad, I.; Tian, E.; Hu, W.; Wang, J.; Wang, B.; Li, R.; Li, J. Colistin-induced autophagy and apoptosis involves the jnk-bcl2-bax signaling pathway and jnk-p53-ros positive feedback loop in pc-12 cells. Chem. Biol. Interact. 2017, 277, 62–73. [Google Scholar] [CrossRef]
- Tian, E.; Hu, W.; Miao, Y.; Muhammad, I.; Zhang, L.; Xia, C.; Ding, L.; Zhang, Q.; Li, R.; Chen, C.; et al. Preventive effects of nerve growth factor against colistin-induced autophagy and apoptosis in pc12 cells. Toxicol. Mech. Methods 2018, 29, 177–186. [Google Scholar] [CrossRef]
- Barta, A.; Janega, P.; Babal, P.; Murar, E.; Cebova, M.; Pechanova, O. The effect of curcumin on liver fibrosis in the rat model of microsurgical cholestasis. Food Funct. 2015, 6, 2187–2193. [Google Scholar] [CrossRef]
- Garcia-Nino, W.R.; Pedraza-Chaverri, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Lu, Z.; Jiang, G.; Chen, Y.; Wang, J.; Muhammad, I.; Zhang, L.; Wang, R.; Liu, F.; Li, R.; Qian, F.; et al. Salidroside attenuates colistin-induced neurotoxicity in rsc96 schwann cells through pi3k/akt pathway. Chem. Biol. Interact. 2017, 271, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Xiong, J.; Wang, Y.; Shen, J.; Velkov, T.; Xiao, X. Nerve growth factor confers neuroprotection against colistin-induced peripheral neurotoxicity. ACS Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Wang, Y.; Sharma, G.; Shen, J.; Velkov, T.; Xiao, X. Polymyxins–Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment. Antioxidants 2020, 9, 506. https://doi.org/10.3390/antiox9060506
Dai C, Wang Y, Sharma G, Shen J, Velkov T, Xiao X. Polymyxins–Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment. Antioxidants. 2020; 9(6):506. https://doi.org/10.3390/antiox9060506
Chicago/Turabian StyleDai, Chongshan, Yang Wang, Gaurav Sharma, Jianzhong Shen, Tony Velkov, and Xilong Xiao. 2020. "Polymyxins–Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment" Antioxidants 9, no. 6: 506. https://doi.org/10.3390/antiox9060506
APA StyleDai, C., Wang, Y., Sharma, G., Shen, J., Velkov, T., & Xiao, X. (2020). Polymyxins–Curcumin Combination Antimicrobial Therapy: Safety Implications and Efficacy for Infection Treatment. Antioxidants, 9(6), 506. https://doi.org/10.3390/antiox9060506