Carotenoids, Fatty Acids, and Volatile Compounds in Apricot Cultivars from Romania—A Chemometric Approach
Abstract
:1. Introduction
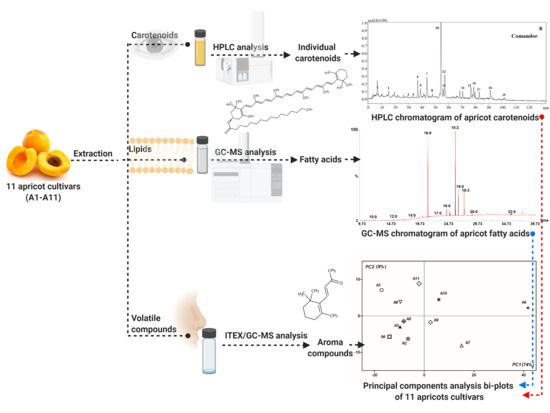
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Carotenoid Extraction
2.4. HPLC-DAD-MS Analysis of Carotenoids
2.5. Lipid Extraction and Fatty Acids Analysis
2.6. Volatile Analysis
2.7. Statistical Analysis
3. Results
3.1. Carotenoids
3.2. Fatty Acids
3.3. Volatiles
3.4. PCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT (Food and Agriculture Organization of United Nations). Statistical Database. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 3 June 2020).
- Topor, E.; Vasilescu, R.; Balan, V.; Tudor, V. Apricot breeding programme for late and very late ripening period in Romania. Acta Hortic. 2010, 862, 137–142. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Cipriano, L.; Nazzaro, F. Apricots: Biochemistry and functional properties. Curr. Opin. Food Sci. 2018, 19, 23–29. [Google Scholar] [CrossRef]
- Aubert, C.; Chanforan, C. Postharvest changes in physicochemical properties and volatile constituents of apricot (Prunus armeniaca L.). Characterization of 28 cultivars. J. Agric. Food Chem. 2007, 55, 3074–3082. [Google Scholar] [CrossRef]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. Characterisation of cell wall polysaccharide profiles of apricots (Prunus armeniaca L.), peaches (Prunus persica L.), and pumpkins (Cucurbita sp.) for the evaluation of fruit product authenticity. Food Chem. 2008, 106, 421–430. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomas-Barberan, F.A.; Gil, M.I. Carotenoids from new apricot (Prunus armeniaca L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical characters and antioxidant, sugar, and mineral nutrient contents in fruit from 29 apricot (Prunus armeniaca L.) cultivars and hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Levaj, B.; Markic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Schmitzer, V.; Slatnar, A.; Mikulic-Petkovsek, M.; Veberic, R.; Krska, B.; Stampar, F. Comparative study of primary and secondary metabolites in apricot (Prunus armeniaca L.) cultivars. J. Sci. Food Agric. 2011, 91, 860–866. [Google Scholar] [CrossRef]
- Cocconi, E.; Stingone, C.; Zanotti, A.; Trifiro, A. Characterization of polyphenols in apricot and peach purees by UHPLC coupled to HRMS Q-ExactiveTM mass spectrometer: An approach in the identification of adulterations. J. Mass Spectrom. 2016, 51, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.; Renard, C.M.G.C.; Reich, M.; Ginies, C.; Audergon, J.M. Change in anthocyanin concentrations in red apricot fruits during ripening. LWT 2009, 42, 372–377. [Google Scholar] [CrossRef]
- Britton, G.; Khachik, F. Carotenoids in food. In Carotenoids. Nutrition and Health; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 2009; Volume 5, pp. 45–66. [Google Scholar]
- Sass-Kiss, A.; Kiss, J.; Milotay, P.; Kerek, M.M.; Toth-Markus, M. Differences in anthocyanin and carotenoid content of fruits and, vegetables. Food Res. Int. 2005, 38, 1023–1029. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Beecher, G.R.; Lusbys, W.R. Separation, Identification, and Quantification of the Major Carotenoids in Extracts of Apricots, Peaches, Cantaloupe, and Pink Grapefruit by Liquid Chromatography. J. Agric. Food Chem. 1989, 37, 1465–1473. [Google Scholar] [CrossRef]
- Kurtz, C.; Carle, R.; Schieber, A. HPLC-DAD-MSn characterisation of carotenoids from apricots and pumpkins for the evaluation of fruit product authenticity. Food Chem. 2008, 110, 522–530. [Google Scholar] [CrossRef]
- Biehler, E.; Alkerwi, A.A.; Hoffmann, L.; Krause, E.; Guillaume, M.; Lair, M.L.; Bohn, T. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J. Food Compos. Anal. 2012, 25, 56–65. [Google Scholar] [CrossRef]
- Iordǎnescu, O.A.; Alexa, E.; Lalescu, D.; Berbecea, C.D.; Poiana, M.A.; Moigradean, D.; Bala, M. Chemical composition and antioxidant activity of some apricot varieties at different ripening stages. Chil. J. Agric. Res. 2018, 78, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Gokbulut, I.; Karabulut, I. SPME–GC–MS detection of volatile compounds in apricot varieties. Food Chem. 2012, 132, 1098–1102. [Google Scholar] [CrossRef]
- Madrau, M.A.; Piscopo, A.; Sanguinetti, A.M.; Del Caro, A.; Poiana, M.; Romeo, F.V.; Piga, A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur. Food Res. Technol. 2009, 228, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Le Bourvellec, C.; Gouble, B.; Bureau, S.; Reling, P.; Bott, R.; Ribas-Agusti, A.; Audergon, J.M.; Renard, C.M.G.C. Impact of canning and storage on apricot carotenoids and polyphenols. Food Chem. 2017, 240, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Erdogan-Orhan, I.; Kartal, M. Insights into research on phytochemistry and biological activities of Prunus armeniaca L. (apricot). Food Res. Int. 2011, 44, 1238–1243. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. Available online: http://www.jbc.org/ (accessed on 30 July 2018). [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Preparation of methyl ester and other derivatives. In Gas Chromatography and Lipids. A Practical Guide, 1st ed.; Christie, W.W., Ed.; Oily Press: Glasgow, UK, 1989; pp. 36–47. [Google Scholar]
- Socaci, S.A.; Socaciu, C.; Tofană, M.; Raţi, I.V.; Pintea, A. In-tube Extraction and GC–MS Analysis of Volatile Components from Wild and Cultivated sea buckthorn (Hippophae rhamnoides L. ssp. Carpatica) Berry Varieties and Juice. Phytochem. Anal. 2013, 24, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Biochem. Biophys. 2018, 648, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. UV/Visible spectroscopy. In Carotenoids Spectroscopy; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1995; Volume 1B, pp. 13–62. [Google Scholar]
- Böhm, V. Use of column temperature to optimize carotenoid isomer separation by C30 high performance liquid chromatography. J. Sep. Sci. 2001, 24, 955–959. [Google Scholar] [CrossRef]
- De Rigal, D.; Gauillard, F.; Richard-Forget, F. Changes in the carotenoid content of apricot (Prunus armeniaca, var Bergeron) during enzymatic browning: β-carotene inhibition of chlorogenic acid degradation. J. Sci. Food Agric. 2000, 80, 763–768. [Google Scholar] [CrossRef]
- Fratianni, A.; Albanese, D.; Mignogna, R.; Cinquanta, L.; Panfili, G.; Di Matteo, M. Degradation of carotenoids in apricot (Prunus armeniaca L.) during drying process. Plant Foods Hum. Nutr. 2013, 68, 241–246. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef]
- Muller, H. Determination of carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection. Z Lebensm Unters Forsch 1997, 204, 88–94. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Bamedi, A. Carotenoid esters in vegetables and fruits: A screening with emphasis on β-cryptoxanthin esters. J. Agric. Food Chem. 2001, 49, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Hacıseferoğulları, H.; Gezer, I.; Özcan, M.M.; Murat Asma, B. Post-harvest chemical and physical–mechanical properties of some apricot varieties cultivated in Turkey. J. Food Eng. 2007, 79, 364–373. [Google Scholar] [CrossRef]
- Duan, Y.; Dong, X.; Liu, B.; Li, P. Relationship of changes in the fatty acid compositions and fruit softening in peach (Prunus persica L. Batsch). Acta Physiol. Plant. 2013, 35, 707–713. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Feng, J.R.; Xi, W.P.; Li, W.H.; Liu, H.N.; Liu, X.F.; Lu, X.Y. Volatile Characterization of Major Apricot Cultivars of Southern Xinjiang Region of China. J. Am. Soc. Hortic. Sci. 2015, 140, 466–471. Available online: http://journal.ashspublications.org/content/140/5/466.full (accessed on 22 July 2018). [CrossRef] [Green Version]
- Xi, W.; Zheng, H.; Zhang, Q.; Li, W. Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef] [Green Version]
- Macleod, G.; Ames, J.M. Volatile components of starfruit. Phytochemistry 1990, 29, 165–172. [Google Scholar] [CrossRef]
- Hallmann, E.; Rozpara, E.; Słowianek, M.; Leszczyńska, J. The effect of organic and conventional farm management on the allergenic potency and bioactive compounds status of apricots (Prunus armeniaca L.). Food Chem. 2019, 279, 171–178. [Google Scholar] [CrossRef]
- Gecer, M.K.; Kan, T.; Gundogdu, M.; Ercisli, S.; Ilhan, G.; Sagbas, H.I. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet. Resour. Crop. Evol. 2020, 67, 935–945. [Google Scholar] [CrossRef]




| ID | Identification | UV-Vis Maxima | MS Data APCI (+) and (−) |
|---|---|---|---|
| 1 | Lutein | 422, 444, 473 | 569 (+); 568 (−) |
| 2 | Phytoene | s276, 286, s297 | 545 (+); 544 (−) |
| 3 | Phytofluene | 333, 347, 367 | 543 (+); 542 (−) |
| 4 | β-Cryptoxanthin | s428, 451, 476 | 553 (+); 552 (−) |
| 5 | β-Carotene-5,8 epoxide | 403, 427, 453 | 553 (+); 552 (−) |
| 6 | 15-cis-β-carotene | 337, 420, 449,472 | 537 (+); 536 (−) |
| 7 | 13 -cis-β-carotene | 338, 420, 444, 469 | 537 (+); 536 (−) |
| 8 | 9,13-di-cis-β-carotene | 336, 421, 444, 473 | 537 (+); 536 (−) |
| 9 | β-Cryptoxanthin-C18:3 | n.d. | 812 (−) |
| 10 | All-trans β-carotene | 421, 452, 478 | 537 (+); 536 (−) |
| 11 | β-Zeacarotene | 402, 426, 450 | 539 (+); 538 (−) |
| 12 | 9-cis-β-carotene | 345, 421, 447, 473 | 537 (+); 536 (−) |
| 13 | β-Cryptoxanthin-C18:2 | n.d. | 814 (−) |
| 14 | β-Cryptoxanthin-C12:0 | s428, 451, 476 | 735 (+); 734 (−) |
| 15 | β-Cryptoxanthin-C18:1 | s428, 451, 476 | 816 (−) |
| 16 | γ-Carotene | 434, 461, 488 | 537 (+); 536 (−) |
| 17 | β-Cryptoxanthin-C16:0 | s428, 450, 476 | 791 (+); 790 (−) |
| 18 | β-Cryptoxanthin-C18:0 | s428, 450, 477 | 819 (+); 818 (−) |
| 19 | Lycopene | 448, 471, 503 | 537 (+); 536 (−) |
| ID | Compound | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lutein | 25.68 ± 1.98d | 54.41 ± 4.81b | 34.36 ± 1.98c | n.d. | 34.99 ± 2.69c | 18.20 ± 1.56e | 129.75 ± 9.19a | 13.95 ± 1.56ef | 41.25 ± 3.68c | 1.69 ± 0.23g | 6.78 ± 0.71fg |
| 2 | Phytoene | 8.56 ± 0.76a | 3.20 ± 0.34d | 3.12 ± 0.34d | 0.50 ± 0.06e | 0.29 ± 0.06e | 0.91 ± 0.24e | 5.90 ± 0.85b | 3.49 ± 0.41cd | 3.17 ± 0.38d | 4.22 ± 0.49c | 6.38 ± 0.58b |
| 3 | Phytofluene | 4.28 ± 0.42b | 3.20 ± 0.38cd | 3.12 ± 0.33d | 0.97 ± 0.14e | 0.58 ± 0.14e | 0.46 ± 0.14e | 5.90 ± 0.92a | 3.49 ± 0.49bcd | 6.35 ± 0.85a | 4.22 ± 0.42bc | 6.38 ± 0.71a |
| 4 | β-Cryptoxanthin | 59.92 ± 5.52d | 137.62 ± 9.62a | 84.35 ± 6.36b | 58.14 ± 4.53d | 46.47 ± 3.11e | 34.13 ± 1.98fg | 11.80 ± 0.95h | 38.35 ± 2.83ef | 60.29 ± 4.24cd | 25.30 ± 1.56g | 70.16 ± 5.52c |
| 5 | β-Carotene-5,8 epoxide | 145.52 ± 9.62f | 99.21 ± 8.91f | 40.61 ± 2.97g | 784.89 ± 70.99a | 124.90 ± 7.21f | 34.13 ± 2.26g | 347.95 ± 18.81d | 247.55 ± 12.73e | 257.02 ± 11.46e | 619.80 ± 31.11b | 465.58 ± 26.87c |
| 6 | 15-cis-β-carotene | 77.04 ± 5.94a | n.d | n.d. | n.d. | 11.62 ± 0.99e | 63.72 ± 4.10b | 5.90 ± 0.99f | 38.35 ± 2.55c | 6.35 ± 0.55f | 29.51 ± 1.64d | 6.38 ± 0.69f |
| 7 | 13 -cis-β-carotene | 479.36 ± 24.32b | 179.22 ± 7.50e | 234.30± 13.72d | n.d. | 110.37 ± 7.21f | 65.99 ± 3.82g | 5.90 ± 0.92h | 226.63 ± 9.90d | 114.23 ± 5.66f | 328.87 ± 15.56c | 854.63 ± 42.43a |
| 8 | 9,13-di-cis-β-carotene | 47.08 ± 4.24c | 19.20 ± 1.70e | 34.36 ± 1.56d | n.d. | 8.71 ± 0.85f | 2.28 ± 0.28gh | 5.90 ± 0.75fg | 24.41 ± 1.70e | 3.17 ± 0.33fgh | 63.24 ± 4.38b | 82.91 ± 5.80a |
| 9 | β-Cryptoxanthin-C18:3 | 0.63 ± 0.08a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d | n.d. | n.d. | n.d. |
| 10 | all-trans-β-carotene | 2456.72 ± 110.31a | 1004.93 ± 39.60e | 952.82 ± 55.15e | 339.15 ± 22.91h | 1002.09 ± 67.88e | 898.85 ± 55.15ef | 536.67 ± 24.47g | 1541.08 ± 66.47c | 786.91 ± 41.01f | 1277.54 ± 56.57d | 1766.65 ± 77.78b |
| 11 | β-Zeacarotene | 17.12 ± 1.27e | 54.41 ± 4.53d | 37.49 ± 2.97d | 7.75 ± 0.52ef | 66.81 ± 6.08b | 100.12 ± 5.94a | 64.87 ± 4.53b | 94.14 ± 7.07a | 60.29 ± 5.37bc | 67.46 ± 5.09b | n.d. |
| 12 | 9-cis-β-carotene | 269.64 ± 14.14cd | 169.62 ± 10.61g | 231.18 ± 11.46ef | 300.39 ± 17.54bc | 246.89 ± 15.56de | 93.30 ± 5.52h | 318.47 ± 16.97b | 198.74 ± 9.90fg | 298.27 ± 12.73bc | 274.06 ± 13.86cd | 644.16 ± 36.77a |
| 13 | β-Cryptoxanthin-C18:2 | 1.89 ± 0.16a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 14 | β-Cryptoxanthin-C12:0 | 27.76 ± 2.05f | 136.21 ± 9.76a | 14.63 ± 0.98g | n.d. | 46.36 ± 3.25e | 45.40 ± 3.25e | 19.61 ± 1.88fg | 102.02 ± 6.22b | 60.50 ± 5.80d | 74.11 ± 5.23c | n.d. |
| 15 | β-Cryptoxanthin-C18:1 | 44.34 ± 3.54d | 350.51 ± 16. 97a | 27.35 ± 1.98e | n.d. | n.d. | n.d. | n.d. | n.d. | 157.09 ± 8.49c | 187.42 ± 11.03b | n.d. |
| 16 | γ-Carotene | 77.04 ± 5. 37d | 169.62 ± 11.31b | 155.09 ± 8.91b | n.d. | 106.48 ± 7.07d | 195.70± 15.56a | 88.46 ± 6.51d | 83.68 ± 7.07d | 22.21 ± 1.84f | 54.81 ± 4.24e | n.d. |
| 17 | β-Cryptoxanthin-C16:0 | 42.84 ± 2.83f | 137.30 ± 10. 18b | 114.99 ± 6.08c | n.d. | 91.38 ± 5.52d | 73.22 ± 4.38e | 21.08 ± 2.12g | 79.77 ± 5.66de | 79.51 ± 5.66de | 181.09 ± 12.73a | n.d. |
| 18 | β-Cryptoxanthin-C18:0 | 21.57 ± 1.56d | 311.85 ± 15.56a | 19.22 ± 1.41d | n.d. | n.d. | n.d. | n.d. | 128.86 ± 7.06b | n.d. | 51.06 ± 3.96c | n.d. |
| 19 | Lycopene | 0.43 ± 0.10d | 3.20 ± 0.30d | 137.06± 11.60a | n.d. | 13.31 ± 2.26c | 36.41 ± 3.82b | n.d | 6.97 ± 0.78cd | 0.74 ± 0.14d | n.d. | n.d. |
| Total carotenoids | 3807.42 ± 194.21a | 2833.70 ± 152.06c | 2124.06 ± 127.79d | 1491.79 ± 116.69f | 1911.25 ± 129.88de | 1662.81 ± 108.00ef | 1568.15 ± 89.84f | 2831.47 ± 142.40c | 1957.34 ± 108.17de | 3244.40 ± 168.11b | 3909.86 ± 197.85a |
| Fatty Acids | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8:0 | 0.02c | 0.02c | 0.05abc | 0.03bc | 0.03bc | 0.08a | 0.06ab | 0.04bc | 0.08a | 0.02c | 0.04bc |
| 10:0 | 0.04bc | 0.03bcd | 0.03bcd | 0.01d | 0.02bcd | 0.09a | 0.05b | 0.02bcd | 0.01cd | 0.03bcd | 0.10a |
| 12:0 | 0.24f | 0.19g | 0.24f | 0.40b | 0.24f | 0.54a | 0.28de | 0.22fg | 0.30d | 0.25ef | 0.33cd |
| 14:0 | 0.30d | 0.28d | 0.35c | 0.08e | 0.27d | 0.35c | 0.53a | 0.27d | 0.27d | 0.27d | 0.40b |
| 15:0 | 0.20a | 0.12e | 0.16bc | 0.17bc | 0.18ab | 0.11e | 0.15cd | 0.13de | 0.13de | 0.20a | 0.12e |
| 16:0 | 29.29abc | 31.13ab | 32.69a | 30.90ab | 29.30abc | 28.69bc | 32.70a | 30.12abc | 30.38abc | 27.27c | 30.44abc |
| 16:1(n-9) | 0.21ab | 0.15de | 0.19b | 0.23a | 0.15de | 0.19b | 0.15de | 0.16cd | 0.20b | 0.13e | 0.14de |
| 16:1(n-7) | 0.18de | 0.08h | 0.17ef | 0.24b | 0.21c | 0.20cd | 0.12g | 0.15f | 0.18de | 0.32a | 0.17ef |
| 16:2(n-4) | 0.18c | 0.07h | 0.13de | 0.26a | 0.04i | 0.12e | 0.13e | 0.10fg | 0.10g | 0.22b | 0.01j |
| 17:0 | 0.42b | 0.21f | 0.32cd | 0.46a | 0.31cd | 0.15g | 0.26e | 0.34c | 0.20f | 0.30d | 0.29de |
| 18:0 | 3.28de | 3.97ab | 3.04efg | 3.13ef | 3.20ef | 2.83fg | 4.15a | 3.78abc | 2.67g | 3.68bcd | 3.41cde |
| 18:1(n-9) | 1.53cd | 1.51cd | 1.36d | 1.40cd | 1.41cd | 1.32d | 1.61c | 1.43cd | 1.36d | 4.64a | 2.38b |
| 18:1(n-7) | 1.73cd | 1.20gh | 1.68de | 1.90bc | 1.61de | 1.51ef | 1.38fg | 1.03h | 1.98b | 2.57a | 1.28g |
| 18:2(n-6) | 46.53a | 46.16a | 43.57a | 42.09a | 44.68a | 43.17a | 44.68a | 42.79a | 46.99a | 45.66a | 43.37a |
| 18:3(n-3) | 14.26c | 12.90cde | 14.30c | 17.16b | 16.66b | 19.11a | 11.47e | 13.40cd | 13.55cd | 12.15de | 14.34c |
| 20:0 | 1.09abc | 1.02bcd | 0.97cde | 0.89ef | 1.10ab | 0.84f | 1.13ab | 1.21a | 0.97cde | 0.96def | 1.04bcd |
| 22:0 | 0.52h | 0.97ef | 0.77fg | 0.64gh | 0.60gh | 0.69gh | 1.14de | 4.81a | 0.64gh | 1.33cd | 2.15b |
| ∑n-3 PUFAs | 14.26c | 12.90cde | 14.30c | 17.16b | 16.66b | 19.11a | 11.47e | 13.40cd | 13.55cd | 12.15de | 14.34c |
| ∑n-6 PUFAs | 46.53a | 46.16 a | 43.57 a | 42.09 a | 44.68 a | 43.17 a | 44.68 a | 42.79 a | 46.99 a | 45.66 a | 43.37 a |
| ∑n-9 PUFAs | 1.74 c | 1.66 c | 1.55 c | 1.63 c | 1.56 c | 1.51 c | 1.76c | 1.59 c | 1.56 c | 4.77a | 2.52b |
| n-6 / n-3 | 3.26cde | 3.58abc | 3.05ef | 2.45gh | 2.68fg | 2.26h | 3.90a | 3.19de | 3.47bcd | 3.76ab | 3.02ef |
| Compound | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||||
| 1-Pentanol | 0.24 ± 0.07d | 0.54 ± 0.06a | 0.35 ± 0.02c | 0.13 ± 0.03e | 0.22 ± 0.08d | 0.21 ± 0.03d | 0.23 ± 0.05d | 0.41 ± 0.16b | 0.35 ± 0.11c | 0.41 ± 0.04b | 0.26 ± 0.06d |
| 3-Pentanol | n.d. | n.d. | n.d. | 0.13 ± 0.04b | 0.14 ± 0.06b | n.d. | 0.16 ± 0.04a | n.d. | n.d. | n.d. | n.d. |
| 3-Hexen-1-ol, (Z)- | 0.50 ± 0.01b | n.d. | 0.67 ± 0.13a | 0.13 ± 0.01f | 0.33 ± 0.05d | 0.51 ± 0.05b | 0.25 ± 0.04e | 0.40 ± 0.05c | 0.72 ± 0.13a | n.d. | n.d. |
| 2-Hexen-1-ol, (E)- | 0.32 ± 0.07a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1-Hexanol | 1.93 ± 0.30a | 1.00 ± 0.28d | 1.51 ± 0.24b | 0.43 ± 0.01fg | 0.32 ± 0.01g | 0.52 ± 0.11f | 0.30 ± 0.11g | 0.70 ± 0.01e | 1.05 ± 0.16d | 0.29 ± 0.01g | 1.29 ± 0.30c |
| Phenol | 0.21 ± 0.06d | 0.64 ± 0.22b | n.d. | 0.51 ± 0.11c | 0.71 ± 0.07a | n.d. | 0.49 ± 0.11c | n.d. | n.d. | 0.76 ± 0.17a | n.d. |
| 1-Hexanol, 2-ethyl- | 0.18 ± 0.01c | n.d. | 0.16 ± 0.02c | 0.04 ± 0.01f | 0.11 ± 0.02d | 0.16 ± 0.01c | 0.11 ± 0.02d | 0.16 ± 0.02c | 0.31 ± 0.12a | 0.25 ± 0.04b | 0.07 ± 0.04e |
| 1-Octanol | 0.06 ± 0.01d | n.d. | n.d. | 0.13 ± 0.03c | 0.29 ± 0.08a | n.d. | 0.19 ± 0.06b | n.d. | n.d. | n.d. | n.d. |
| 1-Nonanol | n.d. | n.d. | n.d. | n.d. | 0.12 ± 0.01a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1-Decanol | n.d. | n.d. | n.d. | n.d. | 0.24 ± 0.04a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Phenol, 2-(1,1-dimethylethyl)-4-methyl- | n.d. | 1.03 ± 0.04d | 1.61 ± 0.32b | 0.91 ± 0.10d | n.d. | 0.52 ± 0.04e | n.d. | 2.74 ± 0.13a | 1.27 ± 0.17c | 1.38 ± 0.21c | n.d. |
| 1-Heptadecanol | n.d. | 0.43 ± 0.09b | n.d. | 1.29 ± 0.16a | n.d. | 0.21 ± 0.02d | n.d. | 0.22 ± 0.01d | n.d. | 0.31 ± 0.04c | n.d. |
| Total | 3.44 ± 0.53b | 3.64 ± 0.69b | 4.3 ± 0.73a | 3.70 ± 0.50b | 2.48 ± 0.42c | 2.13 ± 0.26cd | 1.73 ± 0.43de | 4.63 ± 0.38a | 3.70 ± 0.69b | 3.4 ± 0.51b | 1.62 ± 0.40e |
| Aldehydes | |||||||||||
| Hexanal | 8.06 ± 0.11c | 5.81 ± 1.00de | 9.13 ± 0.02bc | 3.46 ± 0.18f | 8.86 ± 0.45bc | 5.12 ± 0.18e | 6.38 ± 0.3d | 9.41 ± 0.74b | 5.86 ± 0.28de | 16.15 ± 1.12a | 9.70 ± 0.07b |
| Furfural | n.d. | 2.68 ± 0.18a | n.d. | n.d. | n.d. | 0.15 ± 0.04b | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2-Hexenal, (E)- | 0.51 ± 0.09d | 0.5 ± 0.06d | 1.49 ± 0.22b | 0.37 ± 0.05d | 1.43 ± 0.47b | 1.05 ± 0.09c | 0.96 ± 0.06c | 0.42 ± 0.13d | n.d. | 2.63 ± 0.52a | 0.53 ± 0.27d |
| 4-Heptenal, (Z)- | 0.16 ± 0.07f | 0.27 ± 0.08d | 0.51 ± 0.05a | 0.16 ± 0.01f | 0.30 ± 0.11cd | 0.33 ± 0.11bc | 0.21 ± 0.04e | 0.37 ± 0.05b | n.d. | n.d. | 0.26 ± 0.03d |
| Heptanal | 1.42 ± 0.05cd | 2.30 ± 0.28a | 2.53 ± 0.21a | 0.93 ± 0.01e | 1.67 ± 0.15bc | 1.88 ± 0.15b | 1.38 ± 0.08d | 2.45 ± 0.33a | 1.07 ± 0.19e | 2.54 ± 0.13a | 1.94 ± 0.08b |
| 2-Heptenal, (Z)- | n.d. | 0.53 ± 0.18c | 0.48 ± 0.01c | 0.22 ± 0.01e | n.d. | 0.30 ± 0.04d | 0.28 ± 0.1d | 0.60 ± 0.03b | n.d. | 0.67 ± 0.06a | 0.21 ± 0.01e |
| Benzaldehyde | 2.48 ± 0.18a | 1.86 ± 0.11c | 1.43 ± 0.15de | 0.83 ± 0.16h | 1.80 ± 0.30c | 0.92 ± 0.07gh | 1.29 ± 0.07ef | 1.53 ± 0.27d | 1.32 ± 0.20def | 2.15 ± 0.27b | 1.12 ± 0.16fg |
| Octanal | 0.96 ± 0.15d | 1.64 ± 0.06b | 1.27 ± 0.10c | 0.93 ± 0.11d | 1.67 ± 0.40b | 1.19 ± 0.22c | 1.26 ± 0.09c | 1.69 ± 0.06b | 1.20 ± 0.12c | 2.07 ± 0.10a | 0.76 ± 0.23d |
| Benzeneacetaldehyde | n.d. | 0.32 ± 0.17a | n.d. | 0.06 ± 0.01b | n.d. | n.d. | 0.05 ± 0.01b | n.d. | n.d. | n.d. | n.d. |
| 2-Octenal, (E)- | 0.26 ± 0.06e | 0.71 ± 0.06c | 0.86 ± 0.06b | 0.30 ± 0.01e | 0.41 ± 0.05d | 0.69 ± 0.13c | 0.43 ± 0.08d | 1.03 ± 0.11a | n.d. | 0.72 ± 0.04c | 0.48 ± 0.01d |
| 2,5-Furandicarboxaldehyde | n.d. | 1.00 ± 0.10a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Nonanal | 0.87 ± 0.19e | 1.57 ± 0.18b | 1.14 ± 0.28cd | 0.89 ± 0.08e | 1.32 ± 0.30c | 1.20 ± 0.23c | 1.66 ± 0.22b | 1.57 ± 0.39b | 0.95 ± 0.06de | 2.57 ± 0.06a | 0.80 ± 0.24e |
| 5-Hydroxymethylfurfural | n.d. | 4.46 ± 0.28a | n.d. | n.d. | 0.39 ± 0.08d | 1.37 ± 0.27b | n.d. | n.d. | 0.95 ± 0.40c | n.d. | 0.32 ± 0.45d |
| Decanal | 0.37 ± 0.10f | 1.06 ± 0.15b | 0.65 ± 0.14d | 0.50 ± 0.06e | 1.07 ± 0.08b | 0.68 ± 0.06d | 0.88 ± 0.08c | 0.88 ± 0.04c | 0.58 ± 0.08de | 1.37 ± 0.10a | 0.32 ± 0.11f |
| trans-2-Nonenal | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.22 ± 0.01a | n.d. | n.d. | n.d. | n.d. |
| trans-2-Decenal | n.d. | n.d. | n.d. | 0.12 ± 0.01c | 0.28 ± 0.08a | 0.03 ± 0.02d | n.d. | n.d. | n.d. | 0.16 ± 0.02b | n.d. |
| 2-Undecenal | n.d. | 0.56 ± 0.06a | n.d. | 0.27 ± 0.04c | 0.27 ± 0.06c | 0.18 ± 0.01d | 0.38 ± 0.06b | n.d. | n.d. | n.d. | n.d. |
| Octadecanal | n.d. | 0.80 ± 0.21a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Tetradecanal | n.d. | 0.57 ± 0.11a | n.d. | 0.49 ± 0.04b | 0.13 ± 0.05e | 0.19 ± 0.01d | 0.50 ± 0.08b | 0.30 ± 0.04c | 0.10 ± 0.01e | 0.50 ± 0.17b | n.d. |
| Dodecanal | n.d. | n.d. | n.d. | n.d. | n.d. | 0.46 ± 0.08b | 0.40 ± 0.03c | n.d. | n.d. | 0.66 ± 0.18a | n.d. |
| Tridecanal | n.d. | 0.40 ± 0.02b | 0.26 ± 0.06cd | 0.3 ± 0.07c | 0.24 ± 0.03d | 0.16 ± 0.01e | 0.40 ± 0.04b | 0.41 ± 0.11b | n.d. | 0.71 ± 0.10a | n.d. |
| Total | 15.09 ± 1.00d | 27.04 ± 3.29b | 19.75 ± 1.30c | 9.83 ± 0.85e | 19.84 ± 2.61c | 15.9 ± 1.72d | 16.68 ± 1.35d | 20.66 ± 2.30c | 12.03 ± 1.34e | 32.90 ± 2.87a | 16.44 ± 1.64d |
| Ketones | |||||||||||
| 2-Hexanone | n.d. | n.d. | n.d. | n.d. | 0.26 ± 0.07a | 0.10 ± 0.01c | n.d. | n.d. | n.d. | n.d. | 0.13 ± 0.09b |
| 2-Heptanone | n.d. | n.d. | n.d. | 0.06 ± 0.01b | n.d. | n.d. | n.d. | 0.19 ± 0.04a | n.d. | n.d. | n.d. |
| 2-Methyl-6-heptanone | 0.21 ± 0.01c | n.d. | n.d. | 0.09 ± 0.02e | n.d. | 0.10 ± 0.03e | 0.16 ± 0.03d | 0.21 ± 0.05c | n.d. | 0.31 ± 0.06a | 0.28 ± 0.01b |
| 6-methyl-5-Hepten-2-one | 2.92 ± 0.01e | 1.89 ± 0.25f | 4.85 ± 0.29c | 1.34 ± 0.08f | 3.65 ± 0.25d | 1.95 ± 0.08f | 4.69 ± 0.47c | 9.46 ± 0.25a | 4.49 ± 0.49c | 7.33 ± 0.35b | 3.35 ± 0.04de |
| 2,5-Hexanedione | n.d. | n.d. | 0.18 ± 0.04b | n.d. | n.d. | 0.12 ± 0.03bc | 3.09 ± 0.32a | 0.15 ± 0.06b | n.d. | 0.12 ± 0.02bc | n.d. |
| 1,1,3-Trimethyl-2-cyclohexanone | 0.77 ± 0.01b | n.d. | n.d. | 0.07 ± 0.03f | n.d. | 0.13 ± 0.03e | 0.08 ± 0.04ef | 0.52 ± 0.01c | n.d. | 0.28 ± 0.10d | 0.85 ± 0.02a |
| 2-Cyclohexen-1-one, 3-methyl- | 1.18 ± 0.07a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.75 ± 0.16b | 0.29 ± 0.08d | 0.63 ± 0.13c |
| α-Isophorone | 0.42 ± 0.05bc | n.d. | n.d. | n.d. | n.d. | 0.32 ± 0.04c | n.d. | 0.39 ± 0.06b | n.d. | n.d. | 0.45 ± 0.17a |
| Acetophenone | 0.68 ± 0.16e | 1.19 ± 0.10b | 0.84 ± 0.25d | 0.6 ± 0.13e | 1.14 ± 0.06b | 0.56 ± 0.12e | 0.87 ± 0.08cd | 0.95 ± 0.07cd | 0.99 ± 0.13c | 1.37 ± 0.22a | 0.59 ± 0.04e |
| m-Methylacetophenone | 0.22 ± 0.10a | n.d. | n.d. | 0.10 ± 0.01c | n.d. | 0.17 ± 0.04b | n.d. | n.d. | n.d. | n.d. | 0.17 ± 0.06b |
| Total | 6.40 ± 0.41d | 3.08 ± 0.35fg | 5.87 ± 0.58de | 2.26 ± 0.28g | 5.05 ± 0.38e | 3.45 ± 0.38f | 8.89 ± 0.94b | 11.87 ± 0.54a | 6.23 ± 0.78d | 9.70 ± 0.83b | 6.45 ± 0.55d |
| Esters | |||||||||||
| Acetic acid, 2-methylpropyl ester | 0.22 ± 0.01g | 0.77 ± 0.13bc | 0.87 ± 0.36b | 0.26 ± 0.06fg | 0.78 ± 0.21bc | 0.72 ± 0.06c | 0.49 ± 0.09d | 0.83 ± 0.06b | 0.37 ± 0.16e | 1.41 ± 0.04a | 0.36 ± 0.07ef |
| Butanoic acid, 2-methyl-, methyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.12 ± 0.02a | n.d. |
| Butanoic acid, ethyl ester | 1.07 ± 0.05d | 2.73 ± 0.13a | 1.01 ± 0.18d | 1.31 ± 0.28c | 0.90 ± 0.13de | 0.75 ± 0.11e | 1.01 ± 0.41d | 1.43 ± 0.40bc | 1.04 ± 0.3d | 1.51 ± 0.35b | 0.98 ± 0.28d |
| n-Butyl acetate | 4.60 ± 0.16e | 7.75 ± 0.40c | 14.42 ± 3.29a | 4.9 ± 0.51e | 7.15 ± 0.72c | 8.04 ± 0.27c | 5.65 ± 0.67de | 13.69 ± 1.22a | 7.65 ± 0.29c | 11.48 ± 0.84b | 6.89 ± 0.53cd |
| Butanoic acid, 2-oxo-, methyl ester | n.d. | 0.42 ± 0.19cd | 0.30 ± 0.13e | 0.48 ± 0.04bc | 0.79 ± 0.19a | 0.16 ± 0.01f | 0.51 ± 0.06b | 0.33 ± 0.01e | n.d. | 0.39 ± 0.18d | n.d. |
| Butanoic acid, 2-methyl-, ethyl ester | n.d. | 0.68 ± 0.21a | n.d. | 0.15 ± 0.02b | n.d. | n.d. | n.d. | 0.12 ± 0.04c | n.d. | n.d. | 0.08 ± 0.01d |
| 2-Propenoic acid, butyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | 0.06 ± 0.01a | n.d. | n.d. | n.d. | n.d. | n.d. |
| Acetic acid, pentyl ester | n.d. | n.d. | 0.15 ± 0.04a | 0.1 ± 0.02b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hexanoic acid, methyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | 0.10 ± 0.02a | n.d. | n.d. | n.d. | n.d. | n.d. |
| Butanoic acid, butyl ester | 0.30 ± 0.03c | n.d. | 0.50 ± 0.36b | n.d. | n.d. | 0.14 ± 0.04d | n.d. | n.d. | 1.11 ± 0.02a | n.d. | 0.11 ± 0.15d |
| Hexanoic acid, ethyl ester | 0.40 ± 0.08cd | 4.48 ± 0.77a | 0.37 ± 0.01cde | 0.76 ± 0.06b | n.d. | 0.15 ± 0.01fg | n.d. | 0.48 ± 0.01c | n.d. | 0.24 ± 0.13def | 0.20 ± 0.09ef |
| Acetic acid cis-3-hexenyl ester | 1.14 ± 0.18d | 1.05 ± 0.16d | 2.51 ± 0.17b | 1.07 ± 0.06d | 0.07 ± 0.02f | 0.13 ± 0.01ef | 2.96 ± 0.09a | 0.34 ± 0.04e | 2.27 ± 0.43c | 2.05 ± 0.21c | 0.08 ± 0.02f |
| Acetic acid, hexyl ester | 4.01 ± 0.01d | 8.03 ± 0.88b | 11.72 ± 0.25a | 4.25 ± 0.15cd | 0.07 ± 0.01f | 0.35 ± 0.13f | 2.20 ± 0.03e | 0.72 ± 0.11f | 4.88 ± 0.86c | 2.49 ± 0.37e | 1.78 ± 0.45e |
| 2-Hexen-1-ol, acetate, (E)- | 0.73 ± 0.12d | 1.59 ± 0.16b | 1.20 ± 0.08c | 4.17 ± 0.62a | 0.06 ± 0.01e | n.d. | 0.69 ± 0.08d | 0.60 ± 0.01d | 1.17 ± 0.47c | 1.07 ± 0.19c | 0.16 ± 0.01e |
| Propanoic acid, 2-methyl-, 3-methylbutyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | 0.11 ± 0.02a | n.d. | n.d. | n.d. | n.d. | n.d. |
| Propanoic acid, 2-methyl-, 3-hexenyl ester, (Z) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.47 ± 0.04a | n.d. | n.d. | n.d. | n.d. |
| Propanoic acid, 2-methyl-, hexyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.38 ± 0.06a | n.d. | n.d. | n.d. | n.d. |
| Butanoic acid, 2-hexenyl ester, (E)- | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.26 ± 0.07b | n.d. | 0.35 ± 0.10a | n.d. | n.d. |
| Butanoic acid, 3-hexenyl ester, (Z)- | n.d. | n.d. | n.d. | n.d. | 0.42 ± 0.04b | 0.43 ± 0.02b | 0.11 ± 0.01c | n.d. | 0.48 ± 0.13a | n.d. | n.d. |
| Butanoic acid, hexyl ester | n.d. | n.d. | 2.81 ± 0.91a | n.d. | n.d. | 0.16 ± 0.06b | n.d. | n.d. | n.d. | n.d. | n.d. |
| Butanoic acid, 3-methyl-, 3-hexenyl ester, (Z)- | n.d. | n.d. | n.d. | n.d. | n.d. | 0.13 ± 0.05b | 0.95 ± 0.17a | n.d. | n.d. | n.d. | n.d. |
| Hexanoic acid, 3-hexenyl ester, (Z)- | n.d. | n.d. | n.d. | n.d. | n.d. | 0.09 ± 0.03a | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hexanoic acid, hexyl ester | n.d. | n.d. | n.d. | n.d. | n.d. | 0.10 ± 0.01a | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 0.22 ± 0.04e | n.d. | 0.60 ± 0.16bc | n.d. | 2.46 ± 0.15a | 0.50 ± 0.12c | n.d. | 0.67 ± 0.13b | 0.34 ± 0.11d | n.d. | 0.21 ± 0.09e |
| Total | 12.69 ± 0.67f | 27.50 ± 3.03b | 36.46 ± 5.94a | 17.45 ± 1.82de | 12.70 ± 1.48f | 12.12 ± 0.97f | 15.68 ± 1.77e | 19.21 ± 2.02cd | 19.66 ± 2.87cd | 20.76 ± 2.33c | 10.85 ± 1.71f |
| Terpenoids | |||||||||||
| α-Pinene | 0.16 ± 0.01gh | 0.68 ± 0.25a | 0.29 ± 0.06cd | 0.14 ± 0.02g | 0.20 ± 0.04fg | 0.18 ± 0.06fgh | 0.26 ± 0.06de | 0.35 ± 0.16b | 0.20 ± 0.09fg | 0.33 ± 0.11bc | 0.22 ± 0.11ef |
| β-trans-Ocimene | 0.51 ± 0.21b | n.d. | n.d. | 0.06 ± 0.03e | 0.16 ± 0.01d | 0.60 ± 0.07a | n.d. | n.d. | n.d. | n.d. | 0.45 ± 0.06c |
| β-cis-Ocimene | 2.17 ± 0.39a | n.d. | n.d. | 0.47 ± 0.08e | 0.72 ± 0.08d | 1.94 ± 0.08b | 0.45 ± 0.01e | n.d. | 1.56 ± 0.29c | n.d. | 1.71 ± 0.33c |
| β-Pinene | 0.23 ± 0.09d | n.d. | n.d. | 0.16 ± 0.02e | n.d. | 0.27 ± 0.06c | 0.36 ± 0.06b | 0.54 ± 0.14a | 0.40 ± 0.16b | 0.26 ± 0.10cd | 0.15 ± 0.03e |
| β-Myrcene | 2.95 ± 0.09c | 1.57 ± 0.07ef | 1.73 ± 0.30ef | 1.13 ± 0.14g | 1.43 ± 0.29fg | 2.41 ± 0.26d | 1.86 ± 0.13e | n.d. | 3.30 ± 0.41b | 2.81 ± 0.21c | 3.75 ± 0.03a |
| α-Terpinolene | 1.12 ± 0.08a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2-Carene | 1.69 ± 0.22d | 2.30 ± 0.02bc | 2.56 ± 0.36b | 0.84 ± 0.11f | 1.16 ± 0.10e | 1.64 ± 0.22d | 1.48 ± 0.05d | 2.79 ± 0.85a | 2.50 ± 0.47b | 2.14 ± 0.44c | 1.59 ± 0.42d |
| p-Cymene | 0.60 ± 0.04a | n.d. | 0.27 ± 0.08cd | 0.19 ± 0.01fg | 0.15 ± 0.06g | 0.32 ± 0.03c | 0.26 ± 0.05de | 0.20 ± 0.02fg | 0.65 ± 0.01a | 0.21 ± 0.01ef | 0.52 ± 0.06b |
| d-Limonene | 4.01 ± 0.11a | 1.54 ± 0.11cd | 1.39 ± 0.55d | 1.36 ± 0.04d | 1.37 ± 0.23d | 3.34 ± 0.16b | 1.68 ± 0.20cd | 1.47 ± 0.23cd | 4.13 ± 0.14a | 1.77 ± 0.15c | 3.80 ± 0.21a |
| (+)-4-Carene | 1.98 ± 0.40a | n.d. | n.d. | 0.40 ± 0.07e | 0.64 ± 0.04d | 1.66 ± 0.17b | 0.21 ± 0.12f | n.d. | 0.52 ± 0.28de | n.d. | 0.98 ± 0.02c |
| p-Cymenene | 1.46 ± 0.21a | n.d. | n.d. | 0.31 ± 0.11d | 0.29 ± 0.08d | 0.90 ± 0.11c | 0.28 ± 0.06d | n.d. | 1.44 ± 0.25a | n.d. | 1.32 ± 0.26b |
| Benzene, 1-methoxy-4-(1-methylethyl)- | 1.36 ± 0.14a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.91 ± 0.18b | 1.34 ± 0.25a |
| β-Linalool | 25.97 ± 0.25b | 2.89 ± 0.19f | 2.71 ± 0.30f | 9.06 ± 0.40e | 20.33 ± 0.92c | 33.52 ± 1.28a | 16.45 ± 1.21d | 2.53 ± 0.33f | 22 ± 1.45c | 2.01 ± 0.30f | 31.55 ± 1.20a |
| p-Cymen-8-ol | 0.10 ± 0.02b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.12 ± 0.05a |
| α-Terpineol | 4.00 ± 0.02b | 1.13 ± 0.01d | n.d. | n.d. | 3.10 ± 0.02c | 4.76 ± 0.42a | n.d. | 1.13 ± 0.10d | n.d. | n.d. | 4.47 ± 0.06a |
| 1,3,8-p-Menthatriene | 0.39 ± 0.02b | n.d. | n.d. | n.d. | n.d. | 0.45 ± 0.06a | n.d. | n.d. | n.d. | n.d. | 0.34 ± 0.04c |
| β-Cyclocitral | 1.66 ± 0.14a | n.d. | n.d. | n.d. | 0.42 ± 0.11d | n.d. | 0.38 ± 0.06d | 0.60 ± 0.11c | n.d. | 1.00 ± 0.11b | 1.67 ± 0.07a |
| cis-Geraniol | 1.45 ± 0.34bc | n.d. | n.d. | 0.44 ± 0.09e | 1.18 ± 0.02d | 1.39 ± 0.38c | 0.48 ± 0.12e | n.d. | 1.60 ± 0.34b | n.d. | 2.02 ± 0.03a |
| α-Citral | 0.19 ± 0.11a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.13 ± 0.03b |
| Geranylacetone | 0.96 ± 0.14e | 0.83 ± 0.17ef | 2.61 ± 0.13b | 0.63 ± 0.05f | 1.96 ± 0.18c | 0.61 ± 0.03f | 2.19 ± 0.07c | 3.71 ± 0.36a | 0.81 ± 0.02ef | 2.65 ± 0.30b | 1.38 ± 0.12d |
| β-Ionone | 0.94 ± 0.19f | 4.09 ± 0.16c | 2.68 ± 0.12d | 6.84 ± 0.91b | n.d. | 1.35 ± 0.18ef | 1.47 ± 0.19ef | 9.23 ± 0.04a | n.d. | 1.76 ± 0.21e | 1.04 ± 0.21f |
| Dihydro-β-ionone | n.d. | 0.80 ± 0.15c | 1.71 ± 0.17a | 1.44 ± 0.05b | 0.08 ± 0.01e | 0.33 ± 0.03d | n.d. | 1.62 ± 0.12a | 0.33 ± 0.12d | n.d. | n.d. |
| β-Ionol | n.d. | n.d. | 2.11 ± 0.11a | 0.52 ± 0.11d | 0.69 ± 0.03c | n.d. | n.d. | 1.09 ± 0.41b | n.d. | n.d. | n.d. |
| Farnesyl acetone | 0.06 ± 0.01d | n.d. | 0.58 ± 0.01b | n.d. | n.d. | n.d. | n.d. | 0.86 ± 0.15a | n.d. | n.d. | 0.20 ± 0.01c |
| Linalool 3,7-oxide | 0.70 ± 0.16c | n.d. | n.d. | 0.24 ± 0.06e | 0.48 ± 0.11d | 0.96 ± 0.09a | 0.17 ± 0.03e | n.d. | 0.81 ± 0.13b | n.d. | 0.67 ± 0.15c |
| 3-Caranol | n.d. | n.d. | n.d. | 1.21 ± 0.02c | n.d. | n.d. | 1.83 ± 0.28b | n.d. | 6.52 ± 0.55a | n.d. | n.d. |
| Megastigma-4,6(Z),8(E)-triene | n.d. | 3.06 ± 0.35c | 4.18 ± 0.23b | 3.10 ± 0.26c | 1.33 ± 0.30e | 1.84 ± 0.20d | n.d. | 6.39 ± 0.31a | 2.73 ± 0.23c | n.d. | n.d. |
| Total | 54.66 ± 3.39ab | 18.89 ± 1.48fg | 22.82 ± 2.42ef | 28.54 ± 2.58de | 35.69 ± 2.63c | 58.47 ± 3.89a | 29.81 ± 2.7cd | 32.51 ± 3.33cd | 49.5 ± 4.94b | 15.85 ± 2.11g | 59.42 ± 3.75a |
| Lactones | |||||||||||
| Butyrolactone | 0.60 ± 0.17b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.43 ± 0.29a | n.d. | n.d. |
| γ-Dodecalactone | 0.22 ± 0.05ef | 1.01 ± 0.04a | 0.81 ± 0.06c | n.d. | n.d. | n.d. | n.d. | 0.63 ± 0.07d | 0.29 ± 0.05e | 0.89 ± 0.07b | 0.21 ± 0.01f |
| γ-Decalactone | 0.29 ± 0.08f | 1.20 ± 0.13a | 0.90 ± 0.14b | 0.32 ± 0.01f | 0.73 ± 0.08c | 0.30 ± 0.06f | n.d. | 0.89 ± 0.05b | 0.43 ± 0.04e | 0.56 ± 0.21d | 0.36 ± 0.03ef |
| Total | 1.11 ± 0.30d | 2.21 ± 0.17a | 1.71 ± 0.20b | 0.32 ± 0.01f | 0.73 ± 0.08e | 0.30 ± 0.06f | n.d. | 1.52 ± 0.12c | 2.15 ± 0.38a | 1.45 ± 0.28c | 0.57 ± 0.04e |
| Acids | |||||||||||
| Butanoic acid, 3-methyl- | 0.56 ± 0.16a | n.d. | 0.34 ± 0.07b | 0.10 ± 0.02d | n.d. | 0.20 ± 0.02c | 0.11 ± 0.02d | 0.21 ± 0.01c | n.d. | n.d. | n.d. |
| Benzoic Acid | 0.28 ± 0.03b | n.d. | n.d. | n.d. | 0.52 ± 0.06a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Octanoic Acid | n.d. | n.d. | n.d. | n.d. | 0.32 ± 0.03a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Nonanoic acid | n.d. | n.d. | n.d. | n.d. | 0.58 ± 0.06a | n.d. | 0.06 ± 0.01b | n.d. | n.d. | n.d. | n.d. |
| n-Decanoic acid | n.d. | n.d. | n.d. | n.d. | 0.29 ± 0.04a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Dodecanoic acid | n.d. | 1.75 ± 0.17d | n.d. | 7.43 ± 1.29a | 4.43 ± 0.16b | 0.35 ± 0.08e | 2.44 ± 0.64c | 0.44 ± 0.08e | n.d. | 1.67 ± 0.39d | n.d. |
| Tetradecanoic acid | n.d. | 4.68 ± 0.95d | 0.71 ± 0.13ef | 17.72 ± 0.54a | 6.50 ± 0.04c | 1.53 ± 0.21e | 9.27 ± 1.53b | 1.49 ± 0.28e | n.d. | 3.96 ± 0.21d | n.d. |
| Pentadecanoic acid | 0.06 ± 0.01g | 3.89 ± 0.59d | 1.13 ± 0.25f | 9.60 ± 1.15b | 2.91 ± 0.15e | 1.12 ± 0.02f | 11.39 ± 1.36a | 1.47 ± 0.26f | n.d. | 4.95 ± 0.25c | 0.13 ± 0.08g |
| Total | 0.90 ± 0.20fg | 10.32 ± 1.71d | 2.18 ± 0.45ef | 34.85 ± 3.00a | 15.55 ± 0.54c | 3.20 ± 0.33e | 23.27 ± 3.56b | 3.61 ± 0.62e | n.d. | 10.58 ± 0.85d | 0.13 ± 0.08fg |
| Others | |||||||||||
| Benzene, 2-methyl-1,4-bis(1-methylethyl)- | n.d. | 0.42 ± 0.13c | 0.54 ± 0.15b | 0.49 ± 0.08bc | n.d. | n.d. | n.d. | 1.26 ± 0.07a | 0.34 ± 0.23d | n.d. | n.d. |
| Naphthalene, 1-methyl- | 0.22 ± 0.09de | n.d. | n.d. | 0.19 ± 0.08ef | 0.52 ± 0.03bc | 0.22 ± 0.01de | 0.29 ± 0.06d | 0.58 ± 0.09b | 1.27 ± 0.19a | 0.46 ± 0.02c | 0.14 ± 0.01f |
| Naphthalene, 2-methyl- | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.49 ± 0.15a | n.d. | n.d. |
| 2,4,6-Octatriene, 2,6-dimethyl-, (E,Z)- | 1.08 ± 0.08a | n.d. | n.d. | 0.33 ± 0.05d | n.d. | n.d. | 0.25 ± 0.11e | n.d. | 0.82 ± 0.22c | n.d. | 0.94 ± 0.18b |
| Cyclobutene, bis(1-methylethylidene)- | 0.39 ± 0.01b | n.d. | n.d. | 0.11 ± 0.03d | n.d. | 0.47 ± 0.06a | n.d. | n.d. | 0.33 ± 0.05c | n.d. | 0.35 ± 0.13c |
| Total | 1.69 ± 0.18b | 0.42 ± 0.13f | 0.54 ± 0.15ef | 1.12 ± 0.24d | 0.52 ± 0.03ef | 0.69 ± 0.07e | 0.54 ± 0.17ef | 1.84 ± 0.16b | 3.25 ± 0.84a | 0.46 ± 0.02f | 1.43 ± 0.32c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintea, A.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Pop, E.A.; Opriță, V.-A.; Giuffrida, D.; Cacciola, F.; Bartolomeo, G.; Mondello, L. Carotenoids, Fatty Acids, and Volatile Compounds in Apricot Cultivars from Romania—A Chemometric Approach. Antioxidants 2020, 9, 562. https://doi.org/10.3390/antiox9070562
Pintea A, Dulf FV, Bunea A, Socaci SA, Pop EA, Opriță V-A, Giuffrida D, Cacciola F, Bartolomeo G, Mondello L. Carotenoids, Fatty Acids, and Volatile Compounds in Apricot Cultivars from Romania—A Chemometric Approach. Antioxidants. 2020; 9(7):562. https://doi.org/10.3390/antiox9070562
Chicago/Turabian StylePintea, Adela, Francisc Vasile Dulf, Andrea Bunea, Sonia Ancuța Socaci, Elena Andreea Pop, Vlăduț-Alexandru Opriță, Daniele Giuffrida, Francesco Cacciola, Giovanni Bartolomeo, and Luigi Mondello. 2020. "Carotenoids, Fatty Acids, and Volatile Compounds in Apricot Cultivars from Romania—A Chemometric Approach" Antioxidants 9, no. 7: 562. https://doi.org/10.3390/antiox9070562
APA StylePintea, A., Dulf, F. V., Bunea, A., Socaci, S. A., Pop, E. A., Opriță, V. -A., Giuffrida, D., Cacciola, F., Bartolomeo, G., & Mondello, L. (2020). Carotenoids, Fatty Acids, and Volatile Compounds in Apricot Cultivars from Romania—A Chemometric Approach. Antioxidants, 9(7), 562. https://doi.org/10.3390/antiox9070562