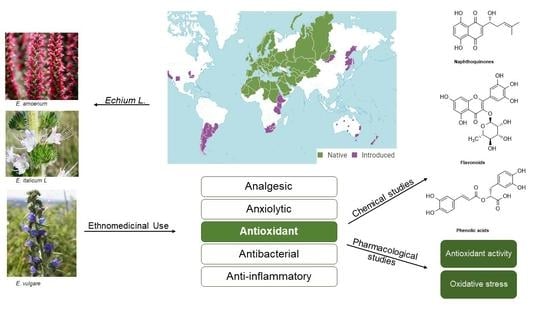
Antioxidant Properties and Reported Ethnomedicinal Use of the Genus Echium (Boraginaceae)
Abstract
:1. Introduction
Aim and Methodology
2. Ethnomedicinal Uses of Echium Species
3. Recorded Antioxidant Activity of the Echium Genus
3.1. Antioxidants
3.2. Oxidative Stress
3.3. Phytochemicals from Echium spp. That Possess Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baltisberger, M.; Widmer, A. Chromosome numbers of plant species from the Canary Islands. Bot. Helv. 2006, 116, 9–30. [Google Scholar] [CrossRef] [Green Version]
- Böhle, U.-R.; Hilger, H.H.; Martin, W.F. Island colonization and evolution of the insular woody habit in Echium L.(Boraginaceae). Proc. Natl. Acad. Sci. USA 1996, 93, 11740–11745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, R.N. Plant Naturalizations and Invasions in the Eastern United States: 1634-1860. Ann. Missouri Bot 2003, 90, 77–90. [Google Scholar] [CrossRef]
- Sayyah, M.; Boostani, H.; Pakseresht, S.; Malaieri, A. Efficacy of aqueous extract of Echium amoenum in treatment of obsessive–compulsive disorder. Prog. NeuroPsychopharmacol. Biol. Psychiatry 2009, 33, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Shafaghi, B.; Naderi, N.; Tahmasb, L.; Kamalinejad, M. Anxiolytic effect of Echium amoenum L. in mice. Iran. J. Pharm. Sci. 2010, 37–41. [Google Scholar]
- Rabbani, M.; Sajjadi, S.E.; Vaseghi, G.; Jafarian, A. Anxiolytic effects of Echium amoenum on the elevated plus-maze model of anxiety in mice. Fitoterapia 2004, 75, 457–464. [Google Scholar] [CrossRef]
- Potdar, V.H.; Kibile, S.J. Evaluation of Antidepressant-like Effect of Citrus Maxima Leaves in Animal Models of Depression. Iran J. Basic Med. Sci. 2011, 14, 478. [Google Scholar]
- Forcella, F.; Wood, J.; Dillon, S. Characteristics distinguishing invasive weeds within Echium (Bugloss). Weed Res. 1986, 26, 351–364. [Google Scholar] [CrossRef]
- Sharma, G.; Esler, K. Phenotypic plasticity among Echium plantagineum populations in different habitats of Western Cape, South Africa. S. Afr. J. Bot 2008, 74, 746–749. [Google Scholar] [CrossRef]
- Konarzewski, T.K.; Murray, B.R.; Godfree, R.C. Rapid development of adaptive, climate-driven clinal variation in seed mass in the invasive annual Forb Echium plantagineum L. PLoS ONE 2012, 7, e49000. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Skoneczny, D.; Weidenhamer, J.D.; Mwendwa, J.M.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Identification and localization of bioactive naphthoquinones in the roots and rhizosphere of Paterson’s curse (Echium plantagineum), a noxious invader. J. Exp. Bot. 2016, 67, 3777–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari, M.R.; Azad, E.M.; Mehrabani, M. Evaluation of the analgesic effect of Echium amoenum Fisch & C.A. Mey. extract in mice: Possible mechanism involved. J. Ethnopharmacol. 2006, 103, 345–349. [Google Scholar] [PubMed]
- Faryadian, S.; Sydmohammadi, A.; Khosravi, A.; Kashiri, M.; Faryadayn, P.; Abasi, N. Aqueous Extract of Echium amoenum Elevate CSF Serotonin and Dopamine Level in Depression rat. Biomed. Pharmacol. J. 2015, 7, 137–142. [Google Scholar] [CrossRef]
- Rabbani, M.; Sajjadi, S.E.; Khalili, S. A Lack of tolerance to the anxiolytic action of Echium amoenum. Res. Pharm. Sci. 2011, 6, 101–106. [Google Scholar]
- Bekhradnia, S.; Ebrahimzadeh, M.A. Antioxidant activity of Echium amoenum. Rev. Chim. J. 2016, 67, 223–226. [Google Scholar]
- Hosseini, N.; Abolhassani, M. Immunomodulatory properties of borage (Echium amoenum) on BALB/c mice infected with Leishmania major. J. Clin. Immunol. 2011, 31, 465–471. [Google Scholar] [CrossRef]
- Abolhassani, M. Antibacterial effect of borage (Echium amoenum) on Staphylococcus aureus. Braz. J. Infect. Dis 2004, 8, 382–385. [Google Scholar] [CrossRef] [Green Version]
- Abolhassani, M. Antiviral activity of borage (Echium amoenum). Arch. Med. Sci. 2010, 6, 366–369. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Paulo, O.S.; Duarte, M.C.; Pina-Martins, F.; Cotrim, M.H.; Carine, M.A.; Pais, M.S. Origin and diversification of the genus Echium (Boraginaceae) in the Cape Verde archipelago. Taxon 2011, 60, 1375–1385. [Google Scholar] [CrossRef]
- El-Shazly, A.; Wink, M. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef] [Green Version]
- Azizi, H.; Ghafari, S.; Ghods, R.; Shojaii, A.; Salmanian, M.; Ghafarzadeh, J. A review study on pharmacological activities, chemical constituents, and traditional uses of Echium amoenum. Pharmacogn. Rev. 2018, 12. [Google Scholar]
- Abbasi, F.; Jamei, R. Effects of Silver Nanoparticles and Silver Nitrate on Antioxidant Responses in Echium amoenum. Russ. J. Plant Physiol. 2019, 66, 488–494. [Google Scholar] [CrossRef]
- Ahvazi, M.; Khalighi-Sigaroodi, F.; Charkhchiyan, M.M.; Mojab, F.; Mozaffarian, V.-A.; Zakeri, H. Introduction of medicinal plants species with the most traditional usage in Alamut region. Iran. J. Pharm. Res. 2012, 11, 185. [Google Scholar] [PubMed]
- Mehrabani, M.; Ghassemi, N.; Ghannadi, E.S.A.; Shams-Ardakani, M. Main phenolic compound of petals of Echium amoenum Fisch. and CA Mey., a famous medicinal plant of Iran. DARU J. Pharm. Sci. 2005, 13, 65–69. [Google Scholar]
- Swamy, M.K.; Patra, J.K.; Rudramurthy, G.R. Medicinal Plants: Chemistry, Pharmacology, and Therapeutic Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- De Natale, A.; Pollio, A. Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy). J. Ethnopharmacol. 2007, 109, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Yilmaz, G.; Kutsal, O.; Yücel, G.; Üstün, O. Bioassay-guided isolation of wound healing active compounds from Echium species growing in Turkey. J. Ethnopharmacol. 2016, 185, 370–376. [Google Scholar] [CrossRef]
- Sezik, E.; Yeşİlada, E.; Tabata, M.; Honda, G.; Takaishi, Y.; Fujita, T.; Tanaka, T.; Takeda, Y. Traditional medicine in Turkey viii. folk medicine in east Anatolia; Erzurum, Erzíncan, Ağri, Kars, Iğdir provinces. Econ. Bot. 1997, 51, 195–211. [Google Scholar] [CrossRef]
- Eruygur, N.; Yilmaz, G.; Üstün, O. Analgesic and antioxidant activity of some Echium species wild growing in Turkey. Fabad J. Pharm. Sci. 2012, 37, 151. [Google Scholar]
- Foster, S.; Duke, J.A. A field guide to medicinal plants: Eastern and central North America. In The Peterson Field Guide Series (USA); Houghton Mifflin Co.: Boston, MA, USA, 1990. [Google Scholar]
- Martín Arroyo, T.; González-Porto, A.V.; Bartolomé Esteban, C. Viper’s bugloss (Echium spp.) honey typing and establishing the pollen threshold for monofloral honey. PLoS ONE 2017, 12, e0185405. [Google Scholar]
- Klemow, K.M.; Clements, D.R.; Threadgill, P.F.; Cavers, P.B. The biology of Canadian weeds. 116. Echium vulgare L. Can. J. Plant Sci. 2002, 82, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Félix-Silva, J.; Silva-Junior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.d.F. Medicinal plants for the treatment of local tissue damage induced by snake venoms: An overview from traditional use to pharmacological evidence. Evid. Based Complementary Altern. Med. 2017, 2017, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Rigat, M.; Vallès, J.; Gras, A.; Iglésias, J.; Garnatje, T. Plants with topical uses in the Ripollès district (Pyrenees, Catalonia, Iberian Peninsula): Ethnobotanical survey and pharmacological validation in the literature. J. Ethnopharmacol. 2015, 164, 162–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef]
- Fujita, T.; Sezik, E.; Tabata, M.; Yesilada, E.; Honda, G.; Takeda, Y.; Tanaka, T.; Takaishi, Y. Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Econ. Bot. 1995, 49, 406. [Google Scholar] [CrossRef]
- Alali, F.Q.; Tahboub, Y.R.; Ibrahim, E.S.; Qandil, A.M.; Tawaha, K.; Burgess, J.P.; Sy, A.; Nakanishi, Y.; Kroll, D.J.; Oberlies, N.H. Pyrrolizidine alkaloids from Echium glomeratum (Boraginaceae). Phytochemistry 2008, 69, 2341–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olennikov, D.; Daironas, Z.V.; Zilfikarov, I. Shikonin and Rosmarinic-Acid Derivatives from Echium russicum Roots. Chem. Nat. Compd. 2017, 53, 953–955. [Google Scholar] [CrossRef]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef] [Green Version]
- Kuete, V.; Wiench, B.; Alsaid, M.S.; Alyahya, M.A.; Fankam, A.G.; Shahat, A.A.; Efferth, T. Cytotoxicity, mode of action and antibacterial activities of selected Saudi Arabian medicinal plants. BMC Complement Altern. Med. 2013, 13, 354. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamaideh, K.D.; El-Elimat, T.; Afifi, F.U.; Kasabri, V. Phytochemical screening and pharmacological activities of Echium judaeum lacaita extracts growing wild in Jordan. Jordan J. Pharm. Sci. 2017, 10, 153–164. [Google Scholar]
- González-Tejero, M.; Molero-Mesa, J.; Casares-Porcel, M.; Lirola, M.M. New contributions to the ethnopharmacology of Spain. J. Ethnopharmacol. 1995, 45, 157–165. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Harraz, F.M.; Al-Ansari, S.M.; El-Mekkawy, S.; Ichino, C.; Kiyohara, H.; Otoguro, K.; Omura, S.; Yamada, H. Antiplasmodial and antitrypanosomal activity of plants from the Kingdom of Saudi Arabia. J. Nat. Med. 2009, 63, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.S.M. Medicinal and Aromatic Plants of Cape Verde: Characterization of Volatile Metabolites of Endemic and Native Species. Ph.D. Thesis, Universidade de Coimbra, Coimbra, Portugal, 2013. [Google Scholar]
- Carvalho, J.C.B.; dos Santos Almeida, H.; Lobo, J.F.R.; Ferreira, J.L.P.; Oliveira, A.P.; Rocha, L. Pyrrolizidine alkaloids in two endemic capeverdian Echium species. Biochem. Syst. Ecol. 2013, 50, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ünsal, Ç.; Vural, H.; Sariyar, G.; Özbek, B.; Ötük, G. Traditional medicine in Bilecik province (Turkey) and antimicrobial activities of selected species. Turk. J. Pharm. Sci. 2010, 7, 139–150. [Google Scholar]
- González-Tejero, M.; Casares-Porcel, M.; Sánchez-Rojas, C.; Ramiro-Gutiérrez, J.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.; Censorii, E.; De Pasquale, C.; Della, A. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F. The protective ability of Mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem. 2009, 112, 587–594. [Google Scholar] [CrossRef]
- Abed, A.; Vaseghi, G.; Jafari, E.; Fattahian, E.; Babhadiashar, N.; Abed, M. Echium Amoenum Fisch. Et Mey: A review on its pharmacological and medicinal properties. Asian J. Med. Pharm. Res. 2014, 4, 21–23. [Google Scholar]
- Pilerood, S.A.; Prakash, J. Evaluation of nutritional composition and antioxidant activity of Borage (Echium amoenum) and Valerian (Valerian officinalis). Int. J. Food Sci. Technol. 2014, 51, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Noroozpour Dailami, K.; Azadbakht, M.; Lashgari, M.; Rashidi, Z. Prevention of selenite-induced cataractogenesis by hydroalchoholic extract of Echium amoenum: An experimental evaluation of the Iranian traditional eye medication. Pharm. Biomed. Res. 2015, 1, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Asghari, B.; Mafakheri, S.; Zarrabi, M.; Erdem, S.; Orhan, I.; Bahadori, M. Therapeutic target enzymes inhibitory potential, antioxidant activity, and rosmarinic acid content of Echium amoenum. S. Afr. J. Bot 2019, 120, 191–197. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Atik-Bekkara, F. Evaluation of antioxidant activity of hydromethanolic extracts of some medicinal species from South Algeria. J. Chin. Med. Assoc. 2014, 77, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, H.; Shams, K.; Mohamed, W.; Ismail, I. Phytochemical and biological investigation of Echium sericeum (Vahl) growing in Egypt. Planta Med. 2007, 73, P_334. [Google Scholar] [CrossRef]
- Dawidar, A.M.; Ghani, A.; Alshamy, M.; Tawfik, E.; Abdel-Mogib, M. Fatty Acid Pattern and alkaloids of Echium rauwolfii. Int. J. Appl. Sci. Eng. 2015, 4, 208–213. [Google Scholar] [CrossRef]
- Kefi, S.; Essid, R.; Mkadmini, K.; Kefi, A.; Haddada, F.M.; Tabbene, O.; Limam, F. Phytochemical investigation and biological activities of Echium arenarium (Guss) extracts. Microb. Pathog. 2018, 118, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Mihailović, V.; Mašković, P.; Solujić, S.; Stojković, A.; Muratspahić, D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef]
- Nadi, F. Bioactive compound retention in Echium amoenum Fisch. & CA Mey. petals: Effect of fluidized bed drying conditions. Int. J. Food Prop. 2017, 20, 2249–2260. [Google Scholar]
- Tahmouzi, S. Extraction, antioxidant and antilisterial activities of polysaccharides from the flower of viper’s bugloss. Int. J. Biol. Macromol. 2014, 69, 523–531. [Google Scholar] [CrossRef]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Stanisławski, G.; Bany, I.; Wójcik, M. Effect of short-term Zn/Pb or long-term multi-metal stress on physiological and morphological parameters of metallicolous and nonmetallicolous Echium vulgare L. populations. Plant Physiol. Biochem. 2017, 115, 380–389. [Google Scholar] [CrossRef]
- Hashemi, Z.; Ebrahimzadeh, M.A.; Khalili, M. Sun protection factor, total phenol, flavonoid contents and antioxidant activity of medicinal plants from Iran. Trop. J. Pharm. Res. 2019, 18, 1443–1448. [Google Scholar]
- Abbaszadeh, S.; Radjabian, T.; Taghizadeh, M. Antioxidant Activity, Phenolic and Flavonoid Contents of Echium Species from Different Geographical Locations of Iran. J. Med. Plants By-Prod. 2013, 2, 23–31. [Google Scholar]
- Tenuta, M.C.; Tundis, R.; Xiao, J.; Loizzo, M.R.; Dugay, A.; Deguin, B. Arbutus species (Ericaceae) as source of valuable bioactive products. Crit. Rev. Food Sci. Nutr. 2019, 59, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Vukajlović, F.N.; Pešić, S.B.; Tanasković, S.T.; Predojević, D.Z.; Gvozdenac, S.M.; Prvulović, D.M.; Bursić, V.P. Efficacy of Echium spp. water extracts as post-harvest grain protectants against Plodia interpunctella. Rom. Biotechnol. Lett. 2018. [Google Scholar]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaeian, L.; Javanmard, S.H.; Ghanadian, M.; Seifabadi, S. Cytoprotective and antioxidant effects of Echium amoenum anthocyanin-rich extract in human endothelial cells (HUVECs). Avicenna J. Phytomed. 2015, 5, 157. [Google Scholar]
- Abbasi Larki, R.; Zayerzadeh, E.; Harzandi, N.; Anissian, A. Protective Effects of Echium amoenum on Oxidative Stress and Gene Expression Induced by Permethrin in Wistar Rats. Hepat Mon. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Abed, A.; Minaiyan, M.; Ghannadi, A.; Mahzouni, P.; Babavalian, M.R. Effect of Echium amoenum Fisch. et Mey a traditional Iranian herbal remedy in an experimental model of acute pancreatitis. ISRN Gastroenterol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kamali, M.; Johari, H. The antioxidant effect of Echium amoenum to prevent teratogenic effects of Lamotrigine on the skeletal system and fetal growth in mice. J. Pharmacol. Clin. Res. 2017, 3, 1–6. [Google Scholar]
- Ranjbar, A.; Khorami, S.; Safarabadi, M.; Shahmoradi, A.; Malekirad, A.A.; Vakilian, K.; Mandegary, A.; Abdollahi, M. Antioxidant activity of Iranian Echium amoenum Fisch & CA Mey flower decoction in humans: A cross-sectional before/after clinical trial. Evid. Based Complementary Altern. Med. 2006, 3, 469–473. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Fazly Bazzaz, B.; Haririzadeh, G.; Imami, S.; Rashed, M. Survey of Iranian plants for alkaloids, flavonoids, saponins, and tannins [Khorasan Province]. Int. J. pharmacogn. 1997, 35, 17–30. [Google Scholar] [CrossRef]
- Chaouche, T.; Haddouchi, F.; Bekkara, F.A. Phytochemical study of roots and leaves of the plant Echium pycnanthum Pomel. Der Pharm. Lett. 2011, 3, 1–4. [Google Scholar]
- Sousa, C.; Moita, E.; Valentão, P.; Fernandes, F.; Monteiro, P.; Andrade, P.B. Effects of colored and noncolored phenolics of Echium plantagineum L. bee pollen in Caco-2 cells under oxidative stress induced by tert-butyl hydroperoxide. J. Agric. Food Chem. 2015, 63, 2083–2091. [Google Scholar] [CrossRef]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid chromatographic–mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated Analysis of COX-2 and iNOS Derived Inflammatory Mediators in LPS-Stimulated RAW Macrophages Pre-Exposed to Echium plantagineum L. Bee Pollen Extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef] [Green Version]
- Ferreres, F.; Pereira, D.M.; Valentão, P.; Andrade, P.B. First report of non-coloured flavonoids in Echium plantagineum bee pollen: Differentiation of isomers by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.G.; Gutiérrez, M.T.; Rial, C.; Torres, A.; Varela, R.M.; Valdivia, M.M.; Molinillo, J.M.; Skoneczny, D.; Weston, L.A.; Macías, F.A. Bioactivity and quantitative analysis of isohexenylnaphthazarins in root periderm of two Echium spp.: E. plantagineum and E. gaditanum. Phytochemistry 2017, 141, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N. A simple isocratic high-perfomance liquid chromatography method for the simultaneous determination of shikonin derivatives in some Echium species growing wild in Turkey. Turk. J. Pharm. Sci. 2018, 15, 38–43. [Google Scholar] [PubMed]
- Dresler, S.; Szymczak, G.; Wójcik, M. Comparison of some secondary metabolite content in the seventeen species of the boraginaceae family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Albreht, A.; Vovk, I.; Simonovska, B.; Srbinoska, M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J. Chromatogr. A 2009, 1216, 3156–3162. [Google Scholar] [CrossRef] [PubMed]
- Zare, K.; Khosrowshahli, M.; Nazemiyeh, H.; Movafeghi, A.; Azar, A.M.; Omidi, Y. Callus culture of Echium italicum L. towards production of a shikonin derivative. Nat. Prod. Res. 2011, 25, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Weston, P.A.; Weston, L.A.; Hildebrand, S. Metabolic profiling in Echium plantagineum: Presence of bioactive pyrrolizidine alkaloids and napthoquinones from accessions across southeastern Australia. Phytochem. Rev. 2013, 12, 831–837. [Google Scholar] [CrossRef]
- Skoneczny, D.; Zhu, X.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Production of pyrrolizidine alkaloids and shikonins in Echium plantagineum L. in response to various plant stressors. Pest Manag. Sci. 2019, 75, 2530–2541. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Tsukada, M.; Mizukami, H.; Tabata, M. Formation of stereoisomeric mixtures of naphthoquinone derivatives in Echium lycopsis callus cultures. Phytochemistry 1983, 22, 453–456. [Google Scholar] [CrossRef]
- Chaouche, T.; Haddouchi, F.; Bekkara, F.A. Identification of shikonin from the roots of Echium pycnanthum Pomel. Asian J. Pharm. Clin. Res. 2012, 5, 30–32. [Google Scholar]
- Dresler, S.; Kubrak, T.; Bogucka-Kocka, A.; Szymczak, G. Determination of shikonin and rosmarinic acid in Echium vulgare L. and Echium russicum JF Gmel. by capillary electrophoresis. J. Liq. Chromatogr. Relat. 2015, 38, 698–701. [Google Scholar] [CrossRef]
- Mitkov, S.; Obreshkova, D.; Ilieva, I.; Pangarova, T.; Pencheva, I. Phenolcarboxylic acids in Echium vulgare L. Acta Pharma. Turcica 2002, 44, 43–48. [Google Scholar]
- Tomás-Barberán, F.A.; Tomás-Lorente, F.; Ferreres, F.; Garcia-Viguera, C. Flavonoids as biochemical markers of the plant origin of bee pollen. J. Sci. Food Agric. 1989, 47, 337–340. [Google Scholar] [CrossRef]
- Pardo, F.; Perich, F.; Torres, R.; Delle Monache, F. Stigmast-4-ene-3, 6-dione an unusual phytotoxic sterone from the roots of Echium vulgare L. Biochem. Syst. Ecol. 2000, 28, 911. [Google Scholar] [CrossRef]
- Dresler, S.; Rutkowska, E.; Bednarek, W.; Stanisławski, G.; Kubrak, T.; Bogucka-Kocka, A.; Wójcik, M. Selected secondary metabolites in Echium vulgare L. populations from nonmetalliferous and metalliferous areas. Phytochemistry 2017, 133, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Oppenheim, J.J.; Howard, O.Z. Cellular pharmacology studies of shikonin derivatives. Phytother. Res. 2002, 16, 199–209. [Google Scholar] [CrossRef]
- Paniagua-Pérez, R.; Madrigal-Bujaidar, E.; Reyes-Cadena, S.; Alvarez-González, I.; Sánchez-Chapul, L.; Pérez-Gallaga, J.; Hernández, N.; Flores-Mondragón, G.; Velasco, O. Cell protection induced by beta-sitosterol: Inhibition of genotoxic damage, stimulation of lymphocyte production, and determination of its antioxidant capacity. Arch. Toxicol. 2008, 82, 615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, A.K.; Dobhal, M.; Sharma, M.; Gupta, R. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef]
| Species | Local Name | Part | Uses | Country |
|---|---|---|---|---|
| E. amoenum Fish. and C.A. Mey. | Gol-e-Gavzaban/Lesan-al-sou | Petals | Demulcent, anti-inflammatory, and analgesic, especially for common cold, pneumonia, anxiolytic, sedative, and other psychiatric symptoms, including obsession [4,23,24,25] | Iran |
| Flower and leaves | Antifebrile, antidepressant, circulatory heart diseases, pulmonary complaints, inflammatory swellings, laxative, emollient [17] | France | ||
| Plant | Heart palpitation [25] | Iran | ||
| Root | Ulcers [25] | Turkey | ||
| Viperina | Aerial part | Depurative, diaphoretic, diuretic, healing respiratory infections [26] | Italy | |
| E. angustifolium Miller. | Kızılcık dikeni, Engerek otu | Roots | Wound healing, ulcer [29] | Turkey |
| E. arabicum R. Mill. | Antiplasmodial and antitrypanosomal activity [27,40] | Saudi Arabia | ||
| E. flavum Desf | Raíz colorá | Root | Antiseptics and wound healing for ulcers and herpes [42] | Spain |
| E. glomeratum Poir. | Sag Al-hamam | Analgesic, diaphoretic, aphrodisiac, snake bites [37] | Jordan | |
| E. hypertropicum Webb. | língua-de-vaca | Seed oil | Cough and gastrointestinal diseases [44,45] | Cape Verde |
| E. italicum L. | Kuşkonmanz/Viperina | Aerial parts | Wound healing, diaphoretic, emollient, diuretic [26,27,35] | Turkey, Italy |
| Dikeni | Leaves | Rheumatic pain, bruises and blisters [35] | Turkey | |
| Roots | Wound healing, ulcer, rheumatic pain, blister, treat bruises [36] | |||
| E. judaeum Lacaita | Lesan Al-Thoor | Hyperactivity, nervousness, general weakness, eczema and dermatological ailments, analgesic, aphrodisiac, diaphoretic [41] | Jordan | |
| E. parviflorum Moench | Kızıl Engerek otu | Aerial parts [27] | Turkey | |
| E. plantagineum L. | Engerek otu | Aerial parts | Diaphoretic and diuretic [46] | Turkey |
| Cough and wound healing [47] | Spain | |||
| E. russicum S. G. Gmel. | Red burgloss | Viper bites [38] | ||
| Havaciva | Root/Vulnerary | Fissures on hand, wound healing [28,39] | Turkey | |
| E. stenosiphon Webb. | língua-de-vaca (cow tongue) | Cough syrup, treatment of cough and gastrointestinal diseases [44,45] | Cape Verde | |
| E. vulcanorum A. Chev. | língua-de-vaca (cow tongue) | Seed oil | Dietetic [44] | Cape Verde |
| E. vulgare L. | Havaciva | Roots | Wound healing, ulcer [27,28,29], bruising, pulled muscles, ligaments and sprains [30] | Turkey, Germany |
| Havaciva Cua de porc | Aerial parts | Diuretic [27,29], snakebites [33,34] | Turkey Spain | |
| Not described | Cough [31] | Spain | ||
| Viper’s bugloss | Leaves, flower | Diuretic [48], bruises and sprains | Germany |
| Plant Name | Plant Part | Constituents Class | Constituents | Analysis Methods |
|---|---|---|---|---|
| E. arenarium (Guss) | Aerial parts [57] | Flavonoids | Luteolin-7-O-glucosides [57] | RP-HPLC [57] |
| Myricetin [57] | ||||
| Quercetin [57] | ||||
| Myricitrin [57] | ||||
| Callus [24] | Phenolic acids | Rosmarinic acid [24] | TLC, HPLC, UV, IR, 1H-NMR, 13C-NMR [81] | |
| E. angustifolium | Root [82] | Naphthoquinones | Shikonin [82] | HPLC-UV [82] |
| Acetylshikonin [82] | ||||
| Deoxyshikonin [82] | ||||
| 3,3-dimethylacrylshikonin [82] | ||||
| 2-methyl-n-butyrylshikonin [82] | ||||
| Isovalerylshikonin [82] | ||||
| E. gaditanum | Root periderm [81] | Shikonin [81] | LC-MS/MS [81] | |
| Acetylshikonin [81] | ||||
| Deoxyshikonin [81] | ||||
| 3,3-dimethylacrylshikonin [81] | ||||
| E. italicum | Shoots, root [83] | Phenolic acids | 4-hydroxybenzoic acid [83] | HPCE [83] |
| Hydrocaffeic acid [83] | ||||
| Rosmarinic acid [83] | ||||
| Root [27,82,83,84] | Naphthoquinones | Acetylshikonin [27,82,84] | HPLC-UV [82,84]; HPCE [83]; TLC, 1H-NMR, 13C-NMR [27]; HPLC-VIS,HPLC-MS, 1H-NMR, 13C-NMR [84] | |
| Angelylshikonin [84] | ||||
| Deoxyshikonin [27,84] | ||||
| Isobutyrylshikonin [84] | ||||
| Isovalerylshikonin [27,82,84] | ||||
| Propionylshikonin [84] | ||||
| Shikonin [27,83,84] | ||||
| Tiglylshikonin [84] | ||||
| 2-methyl-n-butyrylshikonin [27,82,84] | ||||
| 3,3-dimethylacrylshikonin [82] | ||||
| Callus [85] | Naphthoquinones | Shikonin [85] | TLC, HPLC, preparative HPLC, UV, 1H and 13C-NMR [85]; | |
| E. judaeum | Aerial part [41] | Flavonoids | Kaempferol [41] | UPLC-MS [41] |
| Luteolin [41] | ||||
| Aerial part [41] | Phenolic acids | Rosmarinic acid [41] | UPLC-MS [41] | |
| Aerial part [41] | Coumarin | Aesculin [41] | UPLC-MS [41] | |
| E. parviflorum | Root [82] | Naphthoquinones | Acetylshikonin [82,86] | HPLC-UV [82] |
| Deoxyshikonin [82,86] | ||||
| Isovalerylshikonin [82] | ||||
| Shikonin [82,86] | ||||
| 2-methyl-n-butyrylshikonin [82] | ||||
| E. plantagineum | Bee pollen [78,79,80] | Flavonoids | Cyanidin [78] | HPLC-DAD [76,79]; HPLC-PAD-MS; DAD, ESI-MS [78]; |
| Cyanidin-3-(6″-malonylglucoside) [78] | ||||
| Delphinidin [76,78] | ||||
| Isorhamnetin-3-O-rutinoside [80] | ||||
| Kaempferol-3-O-glucoside [76,79,80] | ||||
| Kaempferol-3-O-neohesperidoside [79,80] | ||||
| Kaempferol-3-O-neohesperidoside-7-O-rhamnoside [76,80] | ||||
| Kaempferol-3-O-(4′-rhamnosyl) neohesperidoside [76,79,80] | ||||
| Kaempferol-3-O-(3″/4″acetyl)-neohesperidoside isomer [76,79,80] | ||||
| Kaempferol-3-O-rutinoside [76,79,80] | ||||
| Kaempferol-3-O-sophoroside [76,79,80] | ||||
| Malvidin-3-O-rutinoside [76,78] | ||||
| Peonidin [78] | ||||
| Petunidin-3-O-glucoside [76,78] | ||||
| Petunidin-3-O-rutinoside [78] | ||||
| Quercetin-3-O-sophoroside [80] | ||||
| Quercetin-3-O-neohesperido-side [76,80] | ||||
| Roots and rhizosphere [11,81] | Naphthoquinones | Deoxyshikonin [11,81,87] | LC-ESI/MS [86];LC-MS/MS [81]; UHPLC/Q-ToF MS [11] | |
| Isobutyrylshikonin [87] | ||||
| Isovalerylshikonin [87] | ||||
| Shikonin [11,81,87] | ||||
| Acetylshikonin [11,81] | ||||
| 3,3-dimethylacrylshikonin [11,81,87] | ||||
| 2-methyl-n-butyrylshikonin [87] | ||||
| 3-hydroxyisovalerylshikonin [87] | ||||
| Propionylshikonin [87] | ||||
| Callus [88] | Naphthoquinones | Acetylshikonin [88] | TLC, UV, IR, 1H-NMR, CD [88];PLC-MS-DAD-QToF [87] | |
| Isobutyrylshikonin [88] | ||||
| Isovalerylshikonin [88] | ||||
| 3-hydroxyisovalerylshikonin [88] | ||||
| 3,3-dimethylacrylshikonin [88] | ||||
| E. pycnanthum POMEL | Root [89] | Naphthoquinones | Acetylshikonin [89] | 1H and 13C-NMR [89] |
| Angelylshikonin [89] | ||||
| Isobutyrylshikonin [89] | ||||
| Isovalerylshikonin [89] | ||||
| 3,3-dimethylacrylshikonin [89] | ||||
| 2-methyl-n-butyrylshikonin [89] | ||||
| Propionylshikonin [89] | ||||
| Shikonin [89] | ||||
| Tiglylshikonin [89] | ||||
| E. russicum | Shoots, root [83] | Flavonoids | Rutin [83] | HPCE [83] |
| Shoots [83,90]; Root [38,90] | Phenolic acids | Eritrichin (globoidnan A) [38] | CC, UV, IR and NMR [38]; CZE [90]; HPCE [83] | |
| Lithospermic acid [38] | ||||
| Rosmarinic acid [83,90] | ||||
| Rabdosiin [38] | ||||
| Salvianolic acid A [38] | ||||
| Root [38,83,90] | Naphthoquinones | Acetylshikonin [38] | CC, UV, IR, MS and NMR [38]; CZE [90], HPCE [83]; | |
| Angeloylshikonin [38] | ||||
| deoxyshikonin [38] | ||||
| Isobutylshikonin [38] | ||||
| Isovalerylshikonin [38] | ||||
| Shikonin [83,90] | ||||
| Tigloylshikonin [38] | ||||
| 3-acetoxyisovalerylshikonin [38] | ||||
| 3-hydroxyisovalerylshikonin [38] | ||||
| 3,3-dimethylacrylshikonin [38] | ||||
| E. sericeum (Vahl) | Flavonoids | Apigenin [55] | TLC, HPLC, UV, 1H-NMR and FAB-MS [55]. | |
| Apigenin-7-O-rhamnoside [55] | ||||
| Luteolin-7-O-rutinoside [55] | ||||
| Quercetin-3-O-rhamnoside [55] | ||||
| E. vulgare | Aerial part [91] | Phenolic acids | Caffeic acid [91] | GC [91] |
| Cis- cinnamic acid [91] | ||||
| Ferulic acid [91] | ||||
| p-coumaric acid [91] | ||||
| Shoots [83,90], root [90] | Phenolic acids | Hydrocaffeic acid [83] | CZE [90]; HPCE [83] | |
| Rosmarinic acid [83,90] | ||||
| Chlorogenic acid [83] | ||||
| Shoots [83], bee pollen [92] | Flavonoids | Kaempferol 3-glycoside [92] | HPCE [83]; TLC, 2D PC and HPLC [92] | |
| Quercetin 3-glycoside [92] | ||||
| Rutin [83] | ||||
| Root [82,83,90] | Naphthoquinones | Acetylshikonin [82] | CZE [90]; HPCE [83]; HPLC-UV [82] | |
| Deoxyshikonin [82] | ||||
| Isovalerylshikonin [82] | ||||
| Shikonin [82,83,90] | ||||
| 3,3-dimethylacrylshikonin [82] | ||||
| 2-methyl-n-butyrylshikonin [82] | ||||
| Roots [93] | Sterone | Stigmast-4-ene-3,6-dione [93] | preparative TLC, 1H, 2D-NMR [93] | |
| β-sitosterol [93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Boersch, M.; Nagarajan, A.; Davey, A.K.; Zunk, M. Antioxidant Properties and Reported Ethnomedicinal Use of the Genus Echium (Boraginaceae). Antioxidants 2020, 9, 722. https://doi.org/10.3390/antiox9080722
Jin J, Boersch M, Nagarajan A, Davey AK, Zunk M. Antioxidant Properties and Reported Ethnomedicinal Use of the Genus Echium (Boraginaceae). Antioxidants. 2020; 9(8):722. https://doi.org/10.3390/antiox9080722
Chicago/Turabian StyleJin, Ju, Mark Boersch, Akshaya Nagarajan, Andrew K. Davey, and Matthew Zunk. 2020. "Antioxidant Properties and Reported Ethnomedicinal Use of the Genus Echium (Boraginaceae)" Antioxidants 9, no. 8: 722. https://doi.org/10.3390/antiox9080722
APA StyleJin, J., Boersch, M., Nagarajan, A., Davey, A. K., & Zunk, M. (2020). Antioxidant Properties and Reported Ethnomedicinal Use of the Genus Echium (Boraginaceae). Antioxidants, 9(8), 722. https://doi.org/10.3390/antiox9080722