Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish
Abstract
:1. Introduction
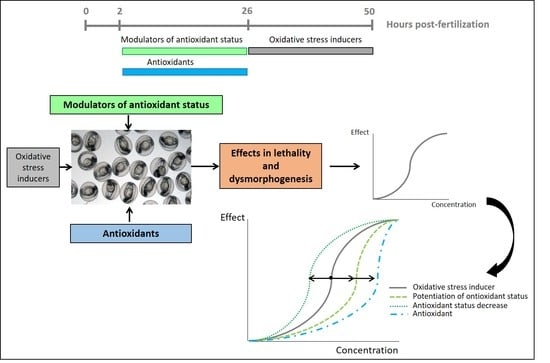
2. Materials and Methods
2.1. Chemicals and Solution Preparation
2.2. Animals and Embryo Production
2.3. Exposure of Zebrafish Embryos to Oxidative Stress Related Compounds
2.4. Pre-Exposure of the Embryos to Modulators of Antioxidant Status + Exposure to OS Inducers
2.5. Pre-Exposure of the Embryos to Antioxidant Compounds + Exposure to OS Inducer
2.6. Data Evaluation
3. Results
3.1. Characterization of the Effects of Oxidative Stress Related Compounds in Zebrafish Embryos
3.2. Pre-Exposure to Modulators of Antioxidant Status + Exposure to OS Inducers
3.3. Detection of Protective Effects of Antioxidant Compounds in Zebrafish Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Becker, K.; Schroecksnadel, S.; Gostner, J.; Zaknun, C.; Schennach, H.; Überall, F.; Fuchs, D. Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine 2014, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Finley, J.W. How to standardize the multiplicity of methods to evaluate natural antioxidants. J. Agric. Food Chem. 2008, 56, 4901–4908. [Google Scholar] [CrossRef] [PubMed]
- Chagas, P.M.; Weber Fulco, B.D.C.; Pesarico, A.P.; Roehrs, J.A.; Wayne, N.C. Bis(phenylimidazolselenazolyl) diselenide as an antioxidant compound: An in vitro and in vivo study. Chem. Biol. Interact. 2015, 233, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speroni, E.; Guerra, M.C.; Minghetti, A.; Crespi-Perellino, N.; Pasini, P.; Piazza, F.; Roda, A. Oleuropein evaluated in vitro and in vivo as an antioxidant. Phyther. Res. 1998, 12, 14–24. [Google Scholar] [CrossRef]
- Phulara, S.C.; Shukla, V.; Tiwari, S.; Pandey, R. Bacopa monnieri promotes longevity in caenorhabditis elegans under stress conditions. Pharmacogn. Mag. 2015, 11, 410–416. [Google Scholar] [PubMed] [Green Version]
- Zhang, Z.J.; Cheang, L.C.V.; Wang, M.W.; Lee, S.M.Y. Quercetin exerts a neuroprotective effect through inhibition of the iNOS/NO system and pro-inflammation gene expression in PC12 cells and in zebrafish. Int. J. Mol. Med. 2011, 27, 195–203. [Google Scholar] [PubMed] [Green Version]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A marvel of high-throughput biology for 21st century. Toxicol. Curr. Environ. Heal. Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [Green Version]
- De Esch, C.; Slieker, R.; Wolterbeek, A.; Woutersen, R.; de Groot, D. Zebrafish as potential model for developmental neurotoxicity testing. A mini review. Neurotoxicol. Teratol. 2012, 34, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.L. Zebrafish: From disease modeling to drug discovery. Curr. Opin. Drug Discov. Devel. 2003, 6, 218–223. [Google Scholar]
- Scholz, S.; Fischer, S.; Gündel, U.; Küster, E.; Luckenbach, T.; Voelker, D. The zebrafish embryo model in environmental risk assessment—Applications beyond acute toxicity testing. Environ. Sci. Pollut. Res. 2008, 15, 394–404. [Google Scholar] [CrossRef]
- Park, K.H.; Cho, K.H. A zebrafish model for the rapid evaluation of pro-oxidative and inflammatory death by lipopolysaccharide, oxidized low-density lipoproteins, and glycated high-density lipoproteins. Fish Shellfish Immunol. 2011, 31, 904–910. [Google Scholar] [CrossRef]
- Kishi, S.; Bayliss, P.E.; Uchiyama, J.; Koshimizu, E.; Qi, J.; Nanjappa, P.; Imamura, S.; Islam, A.; Neuberg, D.; Amsterdam, A.; et al. The identification of zebrafish mutants showing alterations in senescence-associated biomarkers. PLoS Genet. 2008, 4, e1000152. [Google Scholar] [CrossRef] [Green Version]
- Reimers, M.J.; La Du, J.K.; Periera, C.B.; Giovanini, J.; Tanguay, R.L. Ethanol-dependent toxicity in zebrafish is partially attenuated by antioxidants. Neurotoxicol. Teratol. 2006, 28, 497–508. [Google Scholar] [CrossRef]
- Xi, Y.; Noble, S.; Ekker, M. Modeling neurodegeneration in zebrafish. Curr. Neurol. Neurosci. Rep. 2011, 11, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Nakajima-Takagi, Y.; Tsujita, T.; Akiyama, S.I.; Wakasa, T.; Mukaigasa, K.; Kaneko, H.; Tamaru, Y.; Yamamoto, M.; Kobayashi, M. Tissue-restricted expression of Nrf2 and its target genes in zebrafish with gene-specific variations in the induction profiles. PLoS ONE 2011, 6, e26884. [Google Scholar] [CrossRef] [PubMed]
- Timme-Laragy, A.R.; Karchner, S.I.; Franks, D.G.; Jenny, M.J.; Harbeitner, R.C.; Goldstone, J.V.; McArthur, A.G.; Hahn, M.E. Nrf2b, novel zebrafish paralog of oxidant-responsive transcription factor NF-E2-related factor 2 (NRF2). J. Biol. Chem. 2012, 287, 4609–4627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, M.D.; Moreno-Fernández, A.M.; Gomez-Skarmeta, J.L.; de Miguel, M.; Garrido-Maraver, J.; Oropesa-Ávila, M.; Rodríguez-Hernández, Á.; Navas, P.; Sánchez-Alcázar, J.A. Coenzyme Q10 and alpha-tocopherol protect against amitriptyline toxicity. Toxicol. Appl. Pharmacol. 2009, 235, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Seok, S.H.; Baek, M.W.; Lee, H.Y.; Kim, D.J.; Park, S.H.; Lee, H.K.; Park, J.H. Protective effects of vitamin E against 3,3′,4,4’,5-pentachlorobiphenyl (PCB126) induced toxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 2009, 72, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Kais, B.; Schneider, K.E.; Keiter, S.; Henn, K.; Ackermann, C.; Braunbeck, T. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-Implications for the fish embryo test (FET). Aquat. Toxicol. 2013, 140–141, 229–238. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio--a general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar]
- Teixidó, E.; Piqué, E.; Gómez-Catalán, J.; Llobet, J.M. Assessment of developmental delay in the zebrafish embryo teratogenicity assay. Toxicol. Vitr. 2013, 27, 469–478. [Google Scholar] [CrossRef]
- Chen, T.H.; Lin, C.Y.; Tseng, M.C. Behavioral effects of titanium dioxide nanoparticles on larval zebrafish (Danio rerio). Mar. Pollut. Bull. 2011, 63, 303–308. [Google Scholar] [CrossRef]
- Holmberg, A.; Olsson, C.; Holmgren, S. The effects of endogenous and exogenous nitric oxide on gut motility in zebrafish Danio rerio embryos and larvae. J. Exp. Biol. 2006, 209, 2472–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usenko, C.Y.; Harper, S.L.; Tanguay, R.L. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol. Appl. Pharmacol. 2008, 229, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutteridge, J. Free radicals and antioxidant protection: Mechanisms and significance in toxicology and disease. Hum. Toxicol. 1988, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.A.; Murado, M.A. A critical point: The problems associated with the variety of criteria to quantify the antioxidant capacity. J. Agric. Food Chem. 2014, 62, 5472–5484. [Google Scholar] [CrossRef] [Green Version]
- Kanupriya; Prasad, D.; Sai Ram, M.; Sawhney, R.C.; Ilavazhagan, G.; Banerjee, P.K. Mechanism of tert-butylhydroperoxide induced cytotoxicity in U-937 macrophages by alteration of mitochondrial function and generation of ROS. Toxicol. Vitr. 2007, 21, 846–854. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Van Tiem, L.A.; Linney, E.A.; Di Giulio, R.T. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol. Sci. 2009, 109, 217–227. [Google Scholar] [CrossRef]
- Wang, Y.J.; Ho, Y.S.; Chu, S.W.; Lien, H.J.; Liu, T.H.; Lin, J.K. Induction of glutathione depletion, p53 protein accumulation and cellular transformation by tetrachlorohydroquinone, a toxic metabolite of pentachlorophenol. Chem. Biol. Interact. 1997, 105, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lee, C.C.; Chang, W.C.; Liou, H.B.; Ho, Y.S. Oxidative stress and liver toxicity in rats and human hepatoma cell line induced by pentachlorophenol and its major metabolite tetrachlorohydroquinone. Toxicol. Lett. 2001, 122, 157–169. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.H.; Wang, H.; Ji, Y.L.; Ning, H.; Wang, S.F.; Zhang, C.; Lu, J.W.; Duan, Z.H.; Xu, D.X. Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. Toxicol. Sci. 2008, 103, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Kim, E.K.; Jeon, N.J.; Ryu, B.I.; Hwang, J.W.; Choi, E.J.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant effect of Taurine-Rich Paroctopus dofleini extracts through inhibiting ROS production against LPS-induced oxidative stress in vitro and in vivo model. Adv. Exp. Med. Biol. 2017, 975, 1165–1177. [Google Scholar]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priya, S.; Nigam, A.; Bajpai, P.; Kumar, S. Diethyl maleate inhibits MCA+TPA transformed cell growth via modulation of GSH, MAPK, and cancer pathways. Chem Biol Interact. 2014, 219, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J. Biol. Chem. 1982, 257, 13704–13712. [Google Scholar] [PubMed]
- Novoa, B.; Bowman, T.V.; Zon, L.; Figueras, A. LPS response and tolerance in the zebrafish (Danio rerio). Fish Shellfish Immunol. 2009, 26, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, S.; Davis, B.M.; Richens, J.L.; Vere, K.A.; Petrov, P.G.; Winlove, C.P.; O’shea, P. α-Tocopherols modify the membrane dipole potential leading to modulation of ligand binding by P-glycoprotein 4. J. Lipid. Res. 2015, 56, 1543–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008, 60, 13–14. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Invest. Ophthalmol Vis. Sci. 2011, 22, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Rayamajhi, N.; Kim, S.-K.; Go, H.; Joe, Y.; Callaway, Z.; Kang, J.-G.; Ryter, S.; Chung, H. Quercetin induces mitochondrial biogenesis through activation of HO-1 in HepG2 cells. Oxidative Med. Cell. Longev. 2013, 2013, 154279. [Google Scholar] [CrossRef] [Green Version]
- Mourabit, S.; Fitzgerald, J.A.; Ellis, R.P.; Takesono, A.; Porteus, C.S.; Trznadel, M.; Metz, J.; Winter, M.J.; Kudoh, T.; Tyler, C.R. New insights into organ-specific oxidative stress mechanisms using a novel biosensor zebrafish. Environ. Int. 2019, 133, 105138. [Google Scholar] [CrossRef]




| Compounds | Range of Concentrations | MTC | LC50 | EC50 | Exposure Window |
|---|---|---|---|---|---|
| OS Inducers | |||||
| Tert-butyl hydroperoxide (tBOOH) | 1–4 mM | n.d.a | 2.4 mM | 1.6 mM | 26–50 hpf |
| Tetrachlorohydroquinone (TCHQ) | 2.5–20 µM | n.d.a | 16.0 µM | 3.9 µM | 26–50 hpf |
| Lipopolysaccharides from Escherichia coli 0111:B4 (LPS) | 5–60 µg/mL | 25 µg/mL | 50.1 µg/mL | 35.9 µg/mL | 26–50 hpf |
| Modulators of Antioxidant Status | |||||
| n-acetyl-l-cysteine (NAC) | 50–2500 µM | 250 µM | 1874 µM | 920.6 µM | 2–26 hpf |
| Diethyl maleate (DEM) | 0.1–100 µM | 0.5 µM | n.d.b | 1.5 µM | 2–26 hpf |
| Nω-nitro l-arginine methyl ester hydrochloride (L-NAME) | 0.1–100 µM | 5 µM | n.d.c | 44.36 µM | 2–26 hpf |
| DL-buthionine sulfoximine (BSO) | 1–5000 µM | 50 µM | n.d. c | 2722 µM | 2–26 hpf |
| Antioxidants | |||||
| Vit. E | 1–1000 µM | 100 µM | n.d.d | n.d.d | 2–26 hpf |
| Lipoic acid | 0.1–1000 µM | 5 µM | 116.4 µM | n.d.c | 2–26 hpf |
| Quercetin | 0.1–30 µM e | 20 µM | n.d.d | n.d.d | 2–26 hpf |
| Modulator of Antioxidant Status | OS Inducer | LC50 (95% CI) | EC50 (95% CI) |
|---|---|---|---|
| None 1 | Tert-butyl hydroperoxide (tBOOH) | 2.38 mM (2.28–2.48) | 1.64 mM (1.44–1.87) |
| n-acetyl-l-cysteine (NAC) | n.d. | 2.28 mM ** (2.11–2.46) | |
| Nω-nitro l-arginine methyl ester hydrochloride (L-NAME) | n.d. | 3.17 mM *** (2.85–3.52) | |
| Diethyl maleate (DEM) | 2.06 mM * (1.78–2.38) | 1.17 mM ** (1.07–1.29) | |
| DL-buthionine sulfoximine (BSO) | 1.95 mM *** (1.85–2.05) | 1.20 mM * (1.07–1.33) | |
| None | Tetrachlorohydroquinone (TCHQ) | 15.2 µM (13.8–16.7) | 8.84 µM (7.15–10.9) |
| NAC | 19.6 µM * (16.6–23.3) | 15.5 µM *** (14.8-16.3) | |
| L-NAME | 19.0 µM * (17.3–20.9) | 17.1 µM *** (16.9–17.3) | |
| DEM | 9.78 µM ** (7.31–13.1) | 4.79 µM * (3.88–5.91) | |
| BSO | 6.89 µM *** (6.13–7.75) | 4.17 µM ** (3.62–4.81) | |
| None 1 | Lipopolysaccharides from Escherichia coli 0111:B4 (LPS) | 50.1 µg/mL (48.6–51.8) | 36.0 µg/mL (28.4–45.6) |
| NAC | 51.6 µg/mL * (48.8–54.5) | 39.6 µg/mL (35.0–44.8) | |
| L-NAME | 53.4 µg/mL * (51.9–55.0) | 51.3 µg/mL ** (49.6–53.0) | |
| DEM | 42.1 µg/mL *** (37.9–46.8) | 31.1 µg/mL (26.3–36.6) | |
| BSO | 45.2 µg/mL ** (43.2–47.4) | 36.2 µg/mL (29.4–44.5) |
| Antioxidant Compounds | OS Inducer | LC50 (95% CI) | EC50 (95% CI) |
|---|---|---|---|
| None 1 | Tert-butyl hydroperoxide (tBOOH) | 2.38 mM (2.28–2.48) | 1.64 mM (1.44–1.87) |
| Vitamin E | 2.83 mM *** (2.70–2.69) | 2.42 mM *** (2.17–2.70) | |
| Lipoic acid | 3.72 mM *** (3.14–4.40) | 3.70 mM *** (3.03–4.51) | |
| Quercetin | 3.26 mM *** (2.83–3.76) | 3.05 mM *** (2.64–3.54) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boix, N.; Teixido, E.; Pique, E.; Llobet, J.M.; Gomez-Catalan, J. Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants 2020, 9, 721. https://doi.org/10.3390/antiox9080721
Boix N, Teixido E, Pique E, Llobet JM, Gomez-Catalan J. Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants. 2020; 9(8):721. https://doi.org/10.3390/antiox9080721
Chicago/Turabian StyleBoix, Nuria, Elisabet Teixido, Ester Pique, Juan Maria Llobet, and Jesus Gomez-Catalan. 2020. "Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish" Antioxidants 9, no. 8: 721. https://doi.org/10.3390/antiox9080721
APA StyleBoix, N., Teixido, E., Pique, E., Llobet, J. M., & Gomez-Catalan, J. (2020). Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants, 9(8), 721. https://doi.org/10.3390/antiox9080721