Effect of Adding Curcumin on the Properties of Linseed Oil Organogels Used as Fat Replacers in Pâtés
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Linseed Oleogels
2.2.2. Characterization of Organogels Obtained under Optimal Conditions
Polarized Light Microscopy
Mechanical Strength
Oil Binding Capacity
Induction Period
Thermal Analysis
X-ray Diffraction Analysis
Rheological Analysis
Long-Term Oxidation Studies
2.2.3. Oxidative Stability and Fatty Acid Profile of Spreadable Meat Products (pâté)
Formulation of Spreadable Meat Products (pâté)
Fatty Acid Profile of Spreadable Meat Products (pâté)
Thiobarbituric Acid Reactive Substances (TBARs) of Spreadable Meat Products (pâté)
2.2.4. Statistical Analysis
3. Results
3.1. Formulation of Linseed Organogels with Curcumin
3.2. Characterization of Organogels Obtained under Optimal Conditions
3.2.1. Polarized Light Microscopy
3.2.2. Mechanical Strength, OBC and IP
3.2.3. Thermal Analysis
3.2.4. X-ray Diffraction Analysis
3.2.5. Rheological Analysis
3.2.6. Long-Term Oxidation Study
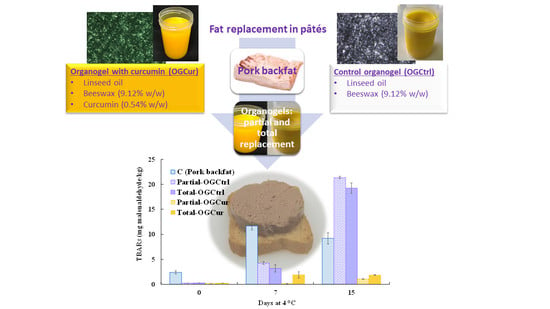
3.3. Oxidative Stability of Spreadable Meat Products (pâté)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sintang, M.D.B.; Danthine, S.; Brown, A.; Van de Walle, D.; Patel, A.R.; Tavernier, I.; Rimaux, T.; Dewettinck, K. Phytosterols-induced viscoelasticity of oleogels prepared by using monoglycerides. Food Res. Int. 2017, 100, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Dewettinck, K. Edible oil structuring: An overview and recent updates. Food Funct. 2016, 7, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marangoni, A.G.; Garti, N. An Overview of the Past, Present, and Future of Organogels, 2nd ed.; AOCS Press: Urbana, IL, USA, 2011; ISBN 9781630670092. [Google Scholar]
- Co, E.D.; Marangoni, A.G. Organogels: An alternative edible oil-structuring method. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar]
- Puscas, A.; Muresan, V.; Socaciu, C.; Muste, S. Oleogels in food: A review of current and potential applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.H.; Reifen, R.; Crawford, M.A. Past and present insights on alpha linolenic acid and the omega-3 fatty acid family. Crit. Rev. Food Sci. Nutr. 2016, 56, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M. A novel propolis wax-based organogel: Effect of oil type on its formation, crystal structure and thermal properties. J. Am. Oil Chem. Soc. 2017, 94, 47–55. [Google Scholar] [CrossRef]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Purriños, L.; Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M.; Zapata, C.; Lorenzo, J.M. Strategy towards replacing pork backfat with a linseed oleogel in Frankfurter sausages and its evaluation on physicochemical, nutritional, and sensory characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Estaca, J.; Herrero, A.M.; Herranz, B.; Álvarez, M.D.; Jiménez-Colmenero, F.; Cofrades, S. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. Assessment of a healthy oil combination structured in ethyl cellulose and beeswax oleogels as animal fat replacers in low-fat, PUFA-enriched pork burgers. Food Bioprocess Technol. 2019, 12, 1068–1081. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. The effect of household storage and cooking practices on quality attributes of pork burgers formulated with PUFA- and curcumin-loaded oleogels as healthy fat substitutes. Food Sci. Technol. 2020, 119, 108909. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Visoli, F.; Franco, M.; Toledo, E.; Luchsinger, J.; Willett, W.C.; Hu, F.B.; Martínez-González, M.A. Olive oil and prevention of chronic diseases: Summary of an international conference. Nutr. Metab. Cardiovas. 2018, 28, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.J.; Cerqueira, M.A.; Cunha, R.L.; Vicente, A.A. Fortified beeswax oleogels: Effect of β-carotene on the gel structure and oxidative stability. Food Funct. 2017, 8, 4241–4250. [Google Scholar] [CrossRef]
- Martinović, N.; Poklar Ulrih, N.; Abramovič, H. Sinapic acid and its derivatives increase oxidative Stability in different model lipid systems. Eur. J. Lipid Sci. Technol. 2019, 121, 1800326. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of curcumin-loaded nanocarriers for food, drug and cosmetic purposes. Trends Food Sci. Technol. 2019, 88, 445–458. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, J.; Chunjiang, L.; Wang, C.; Shen, C.; Chen, Y.; Li, Q. Curcumin and its two analogues improve oxidative stability of fish oil under long-term storage. Eur. J. Lipid Sci. Technol. 2017, 119, 1600105. [Google Scholar] [CrossRef]
- Rege, S.; Momin, S.; Wadekar, S.; Pratap, A.; Bhowmick, D. Effect of demethoxycurcumin and bisdemethoxycurcumin on antioxidant activity of curcumin in refined sunflower oil. J. Food Process. Preserv. 2012, 38, 296–303. [Google Scholar] [CrossRef]
- Rege, S.A.; Momin, S.A. Pro- and antioxidant activity of curcuminoids with lecithin in sunflower oil. Ukr. Food J. 2017, 6, 494–503. [Google Scholar] [CrossRef]
- Banerjee, A.; Ghosh, S.; Ghosh, M. Anti-oxidative effect of turmeric on frying characteristics of soybean oil. J. Food Sci. Technol. 2015, 52, 1760–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Wan, W.; Cheng, W.; Liu, G.; Han, L. Oxidatively stable curcumin-loaded oleogels structured by β-sitosterol and lecithin: Physical characteristics and release behaviour in vitro. Int. J. Food Sci. Technol. 2019, 54, 2502–2510. [Google Scholar] [CrossRef]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012, 131, 48–54. [Google Scholar] [CrossRef]
- O’Sullivan, C.M.; Davidovich-Pinhas, M.; Wright, A.J.; Barbut, S.; Marangoni, A.G. Ethylcellulose oleogels for lipophilic bioactive delivery-effect of oleogelation on: In vitro bioaccessibility and stability of beta-carotene. Food Funct. 2017, 8, 1438–1451. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.L.T.; Arellano, D.B.; Martini, S. Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Res. Int. 2019, 121, 900–909. [Google Scholar] [CrossRef]
- AOCS Official Method Cd 8-53. Peroxide value-acetic acid-chloroform method. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; Firestone, D. (Ed.) American Oil Chemists’ Society: Champaign, IL, USA, 1997; Vol. 1, pp. 147–173. [Google Scholar]
- Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- UNE-EN ISO 5509:200 Aceites y Grasas de Origen Animal y Vegetal Preparación de Ésteres Metílicos de Ácidos Grasos; Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain, 2001.
- Delgado-Pando, G.; Cofrades, S.; Ruiz-Capillas, C.; Triki, M.; Jiménez-Colmenero, F. Enriched n-3 PUFA/konjac gel low-fat pork liver pâté: Lipid oxidation, microbiological properties and biogenic amine formation during chilling storage. Meat Sci. 2012, 92, 762–767. [Google Scholar] [CrossRef]
- Meddour, I.; Yallese, M.; Aouici, H. Investigation and modeling of surface roughness of hard turned AISI 52100 steel: Tool vibration consideration. In Multiphysics Modelling and Simulation for Systems Design and Monitoring. MMSSD 2014. Applied Condition Monitoring; Haddar, M., Abbes, M.S., Choley, J.-Y., Boukharouba, T., Elnady, T., Kanaev, A., Ben Amar, M., Chaari, F., Eds.; Springer: Cham, Switzerland, 2015; Vol. 2, pp. 189–198. [Google Scholar]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Crystallization kinetics of organogels prepared by rice bran wax and vegetable oils. J. Oleo Sci. 2012, 61, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the antioxidant mechanism of curcumin: Classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, Z.; Jafarizadeh-Malmiri, H. Temperature effects on thermodynamic parameters and solubility of curcumin O/W nanodispersions using different thermodynamic models. Int. J. Food Eng. 2019, 15, 1–14. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical properties of rice bran wax in bulk and organogels. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 1163–1173. [Google Scholar] [CrossRef]
- Doan, C.D.; Van De Walle, D.; Dewettinck, K.; Patel, A.R. Evaluating the oil-gelling properties of natural waxes in rice bran oil: Rheological, thermal, and microstructural study. J. Am. Oil Chem. Soc. 2015, 92, 801–811. [Google Scholar] [CrossRef]
- Martini, S.; Tan, C.Y.; Jana, S. Physical characterization of wax/oil crystalline networks. J. Food Sci. 2015, 80, C989–C997. [Google Scholar] [CrossRef] [PubMed]
- Öǧütcü, M.; Yilmaz, E. Oleogels of virgin olive oil with carnauba wax and monoglyceride as spreadable products. Grasas y Aceites 2014, 65, 040. [Google Scholar]
- Suresh, D.; Gurudutt, K.N.; Srinivasan, K. Degradation of bioactive spice compound: Curcumin during domestic cooking. Eur. Food Res. Technol. 2009, 228, 807–812. [Google Scholar] [CrossRef]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M.; Valoppi, F.; Barba, L.; Balducci, C.; Conte, L.; Calligaris, S.; Nicoli, M.C. Pomegranate seed oil organogels structured by propolis wax, beeswax, and their mixture. Eur. J. Lipid Sci. Technol. 2017, 19, 1700032. [Google Scholar] [CrossRef]
- Basson, I.; Reynhardt, E.C. An investigation of the structures and molecular dynamics of natural waxes. III. Montan wax. J. Phys. D Appl. Phys. 1988, 21, 1421–1428. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. JAOCS J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Jana, S.; Martini, S. Physical characterization of crystalline networks formed by binary blends of waxes in soybean oil. Food Res. Int. 2016, 89, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K. Classification of glyceride crystal forms. Acta Chem. Scand. 1966, 20, 2255–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahey, D.A.; Small, D.M.; Kodali, D.R.; Atkinson, D.; Redgrave, T.G. Structure and polymorphism of 1,2-dioleoyl-3-acyl-sn-glycerols. Three- and six-layered structures. Biochemistry 1985, 24, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Ensikat, H.J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Kotel’nikova, E.N.; Platonova, N.V.; Filatov, S.K. Identification of biogenic paraffins and their thermal phase transitions. Geol. Ore Depos. 2007, 49, 697–709. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Boese, M.; Mader, W.; Barthlott, W.; Koch, K. Crystallinity of plant epicuticular waxes: Electron and X-ray diffraction studies. Chem. Phys. Lipids 2006, 144, 45–59. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of chemical composition of two linseed varieties as sources of health-beneficial substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Beddows, C.G.; Jagait, C.; Kelly, M.J. Preservation of alpha-tocopherol in sunflower oil by herbs and spices. Int. J. Food Sci. Nutr. 2000, 51, 327–339. [Google Scholar] [PubMed]
- Zhang, J.J.; Zhang, J.L.; He, S.M.; Wu, K.Z.; Liu, X.D. Thermal studies on the solid-liquid phase transition in binary systems of fatty acids. Thermochim. Acta 2001, 369, 157–160. [Google Scholar] [CrossRef]
- Russell, E.; Lynch, A.; Galvin, K.; Lynch, P.; Kerry, J. Quality of raw, frozen and cooked duck meat as affected by dietary fat α-tocopheryl acetate supplementation. Int. J. Poult. Sci. 2003, 2, 324–334. [Google Scholar]





| Parameters | OGCur | OGCtrl | |||||||
| Curcumin content (g/100 g) | 0.34 ± 0.01 | - | |||||||
| Mechanical strength (N) | 19.51 ± 0.77 a | 19.10 ± 1.16 a | |||||||
| OBC (%) day 1 | 91.4 ± 1.5 a | 90.4 ± 1.4 a | |||||||
| OBC (%) day 7 | 80.4 ± 3.7 a | 80.0 ± 1.2 a | |||||||
| IP (h) | 9.80 ± 0.57 b | 4.37 ± 0.26 a | |||||||
| Protection factor | 2.24 ± 0.13 | - | |||||||
| Samples | TTAGonset | TTAG | ΔHTAG | Tonset | T1 | T2 | T3 | ΔHt | Tgel_X-ray |
| Beeswax heating | - | - | - | 41.2 | 61.7 | 60.2 | 51.1 | 161.9 | - |
| Beeswax cooling | - | - | - | 63.2 | 60.2 | 58.7 | 49.9 | 153.5 | 61.9 |
| OGCtrl heating | −29.1 | −23.8 | 2.8 | 35.6 | 52.7 | 51 | - | 17.8 | - |
| OGCtrl cooling | −9.4 | −18.6 | 1.1 | 54.5 | 51 | 42.6 | 37.3 | 16.5 | 53.5 |
| OGCur heating | −28.7 | −23.8 | 2.5 | 35.7 | 52.7 | 51 | - | 17.1 | - |
| OGCur cooling | −9.3 | −17.9 | 1 | 54.4 | 51.4 | 43.3 | 37.7 | 17.8 | 52.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Carrasco, P.; Paredes-Toledo, J.; Romero-Hasler, P.; Soto-Bustamante, E.; Díaz-Calderón, P.; Robert, P.; Giménez, B. Effect of Adding Curcumin on the Properties of Linseed Oil Organogels Used as Fat Replacers in Pâtés. Antioxidants 2020, 9, 735. https://doi.org/10.3390/antiox9080735
Ramírez-Carrasco P, Paredes-Toledo J, Romero-Hasler P, Soto-Bustamante E, Díaz-Calderón P, Robert P, Giménez B. Effect of Adding Curcumin on the Properties of Linseed Oil Organogels Used as Fat Replacers in Pâtés. Antioxidants. 2020; 9(8):735. https://doi.org/10.3390/antiox9080735
Chicago/Turabian StyleRamírez-Carrasco, Patricia, Javier Paredes-Toledo, Patricio Romero-Hasler, Eduardo Soto-Bustamante, Paulo Díaz-Calderón, Paz Robert, and Begoña Giménez. 2020. "Effect of Adding Curcumin on the Properties of Linseed Oil Organogels Used as Fat Replacers in Pâtés" Antioxidants 9, no. 8: 735. https://doi.org/10.3390/antiox9080735
APA StyleRamírez-Carrasco, P., Paredes-Toledo, J., Romero-Hasler, P., Soto-Bustamante, E., Díaz-Calderón, P., Robert, P., & Giménez, B. (2020). Effect of Adding Curcumin on the Properties of Linseed Oil Organogels Used as Fat Replacers in Pâtés. Antioxidants, 9(8), 735. https://doi.org/10.3390/antiox9080735