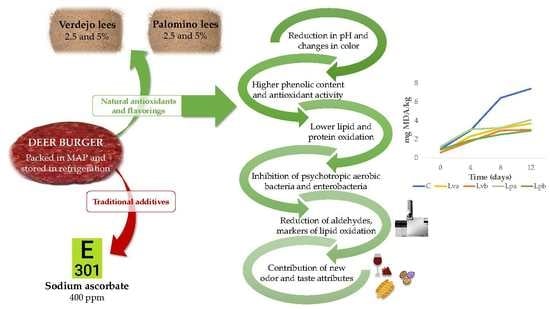
Effect of Wine Lees as Alternative Antioxidants on Physicochemical and Sensorial Composition of Deer Burgers Stored during Chilled Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Ingredients
2.2. Preparation of Burgers and Storage Conditions
2.3. Physicochemical Parameters
2.4. Microbiological Quantification
2.5. Extraction and Analysis of Volatile Compounds
2.6. Sensorial Analysis
2.7. Chemical Characterization of Wine Lees
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters and Color
3.2. Microbiological Quantification
3.3. Total Phenolic Content (TPC) and Radical Scavenging Activity (ABTS, DPPH)
3.4. Lipid and Protein Oxidation
3.5. Volatile Compounds
3.6. Sensorial Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soriano, A.; Murillo, P.; Perales, M.; Sánchez-García, C.; Murillo, J.A.; García Ruíz, A. Nutritional quality of wild Iberian red deer (Cervus elaphus hispanicus) meat: Effects of sex and hunting period. Meat Sci. 2020, in press. [Google Scholar] [CrossRef]
- DeMartini, E.; Vecchiato, D.; Tempesta, T.; Gaviglio, A.; Viganò, R.; Eugenio, D.; Vecchiato, D.; Tiziano, T.; Anna, A.G.; Roberto, V. Consumer preferences for red deer meat: A discrete choice analysis considering attitudes towards wild game meat and hunting. Meat Sci. 2018, 146, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Lund, M.N.; Hviid, M.S.; Skibsted, L.H. The combined effect of antioxidants and modified atmosphere packaging on protein and lipid oxidation in beef patties during chill storage. Meat Sci. 2007, 76, 226–233. [Google Scholar] [CrossRef]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sánchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.; Baenas, N.; Domínguez-Perles, R.; Barros, A.R.; Rosa, E.A.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.G.; Koutinas, A. Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly(3-hydroxybutyrate) synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Jurčević, I.L.; Dora, M.; Guberović, I.; Petras, M.; Brnčić, S.R.; Đikić, D.; Landeka, I.; Rimac, S. Polyphenols from Wine Lees as a Novel Functional Bioactive Compound in the Protection against Oxidative Stress and Hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Andújar-Ortiz, I.; Moreno-Arribas, M.V.; Simo, C.; González, J.; Chana, A.; Dávalos, J.Z.; Ángeles Pozo-Bayón, M. Impact of Glutathione-Enriched Inactive Dry Yeast Preparations on the Stability of Terpenes during Model Wine Aging. J. Agric. Food Chem. 2014, 62, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-Y.; Shyu, Y.-S.; Hsu, C.-K. Grape wine lees improves the rheological and adds antioxidant properties to ice cream. LWT Food Sci. Technol. 2009, 42, 312–318. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, R.; Azad, Z.R.A.A.; Adsule, P.G. Use of fine wine lees for value addition in ice cream. J. Food Sci. Technol. 2015, 52, 592–596. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Selani, M.M.; Contreras-Castillo, C.J.; Shirahigue, L.D.; Gallo, C.R.; Plata-Oviedo, M.S.; Montes-Villanueva, N.D. Wine industry residues extracts as natural antioxidants in raw and cooked chicken meat during frozen storage. Meat Sci. 2011, 88, 397–403. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-San José, M.L.; Del Pino-García, R.; Rivero-Pérez, M.D.; Muñiz-Rodríguez, P. Antioxidant and Antimicrobial Properties of Wine Byproducts and Their Potential Uses in the Food Industry. J. Agric. Food Chem. 2014, 62, 12595–12602. [Google Scholar] [CrossRef] [Green Version]
- Soriano, A.; Alañón, M.E.; Alarcón, M.; García-Ruíz, A.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Oak wood extracts as natural antioxidants to increase shelf life of raw pork patties in modified atmosphere packaging. Food Res. Int. 2018, 111, 524–533. [Google Scholar] [CrossRef]
- Serrano, A.; Cofrades, S.; Jiménez-Colmenero, F. Characteristics of restructured beef steak with different proportions of walnut during frozen storage. Meat Sci. 2006, 72, 108–115. [Google Scholar] [CrossRef]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: Influence on colour and texture deterioration during chill storage. Meat Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef]
- Hierro, E.; De La Hoz, L.; Ordóñez, J.A. Headspace volatile compounds from salted and occasionally smoked dried meats (cecinas) as affected by animal species. Food Chem. 2004, 85, 649–657. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef]
- Vivas, N.; Glories, Y.; Lagune, L.; Saucier, C.; Augustin, M. Estimation du degré de polymérisation des procyanidines du raisin et du vin par la méthode au p-dimethylaminocinnamaldéhyde. OENO One 1994, 28, 319. [Google Scholar] [CrossRef]
- Yin, M.C.; Faustman, C. Influence of temperature, pH, and phospholipid composition upon the stability of myoglobin and phospholipid: A liposome model. J. Agric. Food Chem. 1993, 41, 853–857. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M.L. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef]
- Vergara, H.; Gallego, L.; García, A.J.; Landete-Castillejos, T. Conservation of Cervus elaphus meat in modified atmospheres. Meat Sci. 2003, 65, 779–783. [Google Scholar] [CrossRef]
- Soriano, A.; Montoro, V.; Vicente, J.; Sánchez-Migallón, B.F.; Benítez, S.; Utrilla, M.C.; García Ruiz, A. Influence of evisceration time and carcass ageing conditions on wild venison quality. Preliminary study. Meat Sci. 2016, 114, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Chin, K.B. Evaluation of Antioxidative Activity of Various Levels of Ethanol Extracted Tomato Powder and Application to Pork Patties. Korean J. Food Sci. Anim. Resour. 2017, 37, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Chouliara, E.; Karatapanis, A.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 °C. Food Microbiol. 2007, 24, 607–617. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Szendrő, Z.; Fratini, F.; Paci, G. Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on physical characteristics and oxidative status of fresh and stored rabbit burgers. Meat Sci. 2015, 110, 93–100. [Google Scholar] [CrossRef]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef]
- Primo, E. Oleaginosas. Grasas Animales. Grasas Plásticas. Química de los Alimentos; Editorial Síntesis: Madrid, Spain, 1998; pp. 195–202. [Google Scholar]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L.; Skibsted, L.H.; Jongberg, S. Effect of skin wine pomace and sulfite on protein oxidation in beef patties during high oygen atmosphere storage. Food Bioprocess Technol. 2016, 9, 532–542. [Google Scholar] [CrossRef] [Green Version]
- Ergezer, H.; Serdaroğlu, M. Antioxidant potential of artichoke (Cynara scolymus L.) byproducts extracts in raw beef patties during refrigerated storage. J. Food Meas. Charact. 2018, 12, 982–991. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Olivares, A.; Dryahina, K.; Španěl, P.; Flores, M. Rapid detection of lipid oxidation in beef muscle packed under modified atmosphere by measuring volatile organic compounds using SIFT-MS. Food Chem. 2012, 135, 1801–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, Y.; Watanabe, A.; Shingu, H.; Kushibiki, S.; Hodate, K.; Ishida, M.; Ikeda, S.; Takeda, T. Effects of α-tocopherol level in raw venison on lipid oxidation and volatiles during storage. Meat Sci. 2002, 62, 457–462. [Google Scholar] [CrossRef]
- Shahidi, F. Flavor of meat and meat products, an overview. In Flavor of Meat and Meat Products; Shahidi, F., Ed.; Blackie Academic and Professional: London, UK, 1994; pp. 1–3. [Google Scholar]
- García-Lomillo, J.; González-San José, M.L.; Del Pino-García, R.; Ortega-Heras, M.; Muñiz-Rodríguez, P. Antioxidant effect of seasonings derived from wine pomace on lipid oxidation in refrigerated and frozen beef patties. LWT Food Sci. Technol. 2017, 77, 85–91. [Google Scholar] [CrossRef]
- DeMan, J.H. Lipids in Principles of Food Chemistry, 2nd ed.; Van Nostrand Reinhold: New York, NY, USA, 1990. [Google Scholar]
- Marco, A.; Navarro, J.L.; Flores, M. Quantitation of Selected Odor-Active Constituents in Dry Fermented Sausages Prepared with Different Curing Salts. J. Agric. Food Chem. 2007, 55, 3058–3065. [Google Scholar] [CrossRef]
- Paleari, M.A.; Moretti, V.M.; Beretta, G.; Mentasti, T.; Bersani, C. Cured products from different animal species. Meat Sci. 2003, 63, 485–489. [Google Scholar] [CrossRef]
- Price, A.; Díaz, P.; Bañón, S.; Garrido, M.D. Natural extracts versus sodium ascorbate to extend the shelf life of meat-based ready-to-eat meals. Food Sci. Technol. Int. 2013, 19, 427–438. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañón, S. Use of dietary rosemary diterpenes to inhibit rancid volatiles in lamb meat packed under protective atmosphere. Animal 2016, 10, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Andrés, A.I.; Cava, R.; Ventanas, S.; Muriel, E.; Ruiz, J.; Ruiz-Carrascal, J. Effect of salt content and processing conditions on volatile compounds formation throughout the ripening of Iberian ham. Eur. Food Res. Technol. 2007, 225, 677–684. [Google Scholar] [CrossRef]
- Sabio, E.; Vidal-Aragón, M.C.; Bernalte, M.J.; Gata, J.L. Volatile compounds present in six types of dry-cured ham from south European countries. Food Chem. 1998, 61, 493–503. [Google Scholar] [CrossRef]
- Ramírez, R.; Cava, R. Volatile Profiles of Dry-Cured Meat Products from Three Different Iberian X Duroc Genotypes. J. Agric. Food Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Córdoba, J.J.; Aranda, E.; Córdoba, M.G.; Asensio, M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006, 110, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carpena, J.-G.; Morcuende, D.; Estévez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef] [PubMed]
 C;
C;  Lva;
Lva;  Lvb;
Lvb;  Lpa;
Lpa;  Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees.
Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees.
 C;
C;  Lva;
Lva;  Lvb;
Lvb;  Lpa;
Lpa;  Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees.
Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees.


 C;
C;  Lva;
Lva;  Lvb;
Lvb;  Lpa;
Lpa;  Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees. (A) Raw burgers: Appearance and odor attributes; (B) Cooked burgers: Appearance and odor attributes; (C) Cooked burgers: Taste and texture attributes.
Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees. (A) Raw burgers: Appearance and odor attributes; (B) Cooked burgers: Appearance and odor attributes; (C) Cooked burgers: Taste and texture attributes.
 C;
C;  Lva;
Lva;  Lvb;
Lvb;  Lpa;
Lpa;  Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees. (A) Raw burgers: Appearance and odor attributes; (B) Cooked burgers: Appearance and odor attributes; (C) Cooked burgers: Taste and texture attributes.
Lpb. C: sodium ascorbate control; Lva: 2.5% “verdejo” lees; Lvb: 5% “verdejo” lees; Lpa: 2.5% “palomino” lees; Lpb: 5% “palomino” lees. (A) Raw burgers: Appearance and odor attributes; (B) Cooked burgers: Appearance and odor attributes; (C) Cooked burgers: Taste and texture attributes.
| Lv | Lp | |
|---|---|---|
| Volatile Compounds (µg/gDM) | ||
| ∑ esters | 199.35 ± 32.70 | 199.60 ± 62.36 |
| ∑ acids | 26.77 ± 8.46 | 18.56 ± 7.14 |
| ∑ alcohols | 1.34 ± 0.43 | 1.27 ± 0.55 |
| ∑ aldehydes | 0.65 ± 0.08 | 0.56 ± 0.19 |
| ∑ terpenes and C13 norisoprenoids | 0.40 ± 0.05 a | 0.67 ± 0.07 b |
| ∑ furanic compounds | 0.64 ± 0.25 | 0.47 ± 0.19 |
| Phenolic Composition | ||
| Hydroxycinnamic acid derivates (mg HCA/100 gDM) | 1.53 ± 0.11 a | 2.59 ± 0.12 b |
| Flavonols (mg quercetin/100 gDM) | 1.45 ± 0.12 a | 2.54 ± 0.12 b |
| Catechins (mg catechin/100 gDM) | 15.09 ± 1.36 a | 30.24 ± 1.71 b |
| C | Lva | Lvb | Lpa | Lpb | |
|---|---|---|---|---|---|
| pH | |||||
| Day 0 | 5.66 ± 0.01 d,w | 5.22 ± 0.08 c,x | 4.78 ± 0.02 a,y | 5.16 ± 0.02 c,w | 4.91 ± 0.02 b,x |
| 4 | 5.45 ± 0.01 c,x | 5.14 ± 0.02 b,x | 4.94 ± 0.07 a,x | 5.12 ± 0.01 b,x | 4.90 ± 0.07 a,x |
| 8 | 5.19 ± 0.02 c,y | 4.86 ± 0.02 b,y | 4.70 ± 0.07 a,y | 4.80 ± 0.02 a,b,y | 4.73 ± 0.08 a,y |
| 12 | 5.03 ± 0.02 c,z | 4.73 ± 0.04 b,z | 4.54 ± 0.02 a,z | 4.75 ± 0.01 b,z | 4.55 ± 0.05 a,z |
| Moisture (%) | |||||
| Day 0 | 69.65 ± 1.02 c | 66.37 ± 0.00 a,b | 65.21 ± 0.83 a | 67.71 ± 0.90 b | 65.04 ± 0.56 a |
| 4 | 67.22 ± 1.03 | 67.02 ± 0.68 | 66.36 ± 0.33 | 68.10 ± 0.68 | 66.28 ± 0.83 |
| 8 | 67.85 ± 0.65 b | 66.84 ± 0.29 a,b | 65.14 ± 1.41 a | 66.20 ± 0.88 a,b | 65.70 ± 0.61 a |
| 12 | 69.63 ± 1.47 c | 67.18 ± 0.26 b | 64.86 ± 0.65 a | 67.00 ± 0.57 b | 66.13 ± 0.12 a,b |
| L* | |||||
| Day 0 | 47.60 ± 0.75 a,z | 48.72 ± 0.34 a,z,y | 51.28 ± 1.44 b,z,y | 47.21 ± 1.73 a,z | 51.44 ± 0.88 b,y |
| 4 | 49.49 ± 1.36 a,b,z | 50.79 ± 1.04 b,y | 52.94 ± 0.46 c,y | 48.73 ± 0.26 a,z,y | 51.13 ± 0.72 b,y |
| 8 | 48.60 ± 2.08 z | 47.44 ± 1.27 z | 48.80 ± 1.04 z | 47.14 ± 1.56 z | 48.48 ± 1.50 z |
| 12 | 53.63 ± 2.58 y | 50.32 ± 0.66 y | 52.67 ± 2.34 y | 50.64 ± 0.83 y | 52.57 ± 0.77 y |
| a* | |||||
| Day 0 | 13.30 ± 0.96 c,x | 7.27 ± 0.41 a,b,y | 6.38 ± 0.18 a,y | 7.60 ± 0.22 b,y | 6.26 ± 0.41 a,y |
| 4 | 7.90 ± 0.60 c,y | 5.41 ± 0.51 a,b,z | 5.05 ± 0.31 a,z | 6.24 ± 0.25 b,z | 6.00 ± 0.12 b,y |
| 8 | 4.90 ± 0.53 z | 4.85 ± 1.00 z | 4.95 ± 0.09 z | 6.15 ± 0.45 z | 4.40 ± 1.10 z |
| 12 | 4.65 ± 0.15 a,z | 6.00 ± 0.25 b,z | 4.99 ± 0.91 a,b,z | 5.62 ± 0.46 a,b,z | 5.46 ± 0.07 a,b,z,y |
| b* | |||||
| Day 0 | 19.75 ± 0.58 y | 19.77 ± 0.56 y | 20.13 ± 0.86 z | 19.66 ± 0.93 z | 20.82 ± 0.73 y |
| 4 | 17.64 ± 0.64 a,b,z | 17.07 ± 0.77 a,z | 19.14 ± 0.52 b,z | 18.18 ± 0.59 a,b,z | 18.96 ± 0.47 b,z |
| 8 | 27.18 ± 0.53 b,x | 26.27 ± 0.47 b,x | 27.13 ± 0.63 b,y | 24.69 ± 0.61 a,y | 26.89 ± 0.80 b,x |
| 12 | 20.18 ± 0.61 b,y | 18.05 ± 0.47 a,z | 19.92 ± 0.77 b,z | 18.87 ± 0.59 a,b,z | 19.38 ± 0.40 b,z |
| C | Lva | Lvb | Lpa | Lpb | |
|---|---|---|---|---|---|
| TBARs (mg MDA/kg) | |||||
| Day 0 | 0.93 ± 0.18 b,z | 0.54 ± 0.06 a,z | 0.58 ± 0.07 a,z | 1.25 ± 0.17 c,z | 0.92 ± 0.14 b,z |
| 4 | 2.95 ± 0.36 c,y | 2.32 ± 0.36 b,y | 1.77 ± 0.11 a,y | 3.05 ± 0.25 c,y | 1.90 ± 0.13 a,y |
| 8 | 6.42 ± 0.66 b,x | 3.15 ± 0.64 a,x | 2.93 ± 0.17 a,x | 3.20 ± 0.21 a,y | 2.50 ± 0.31 a,x |
| 12 | 7.39 ± 0.91 c,w | 3.67 ± 0.26 a,b,w | 3.01 ± 0.41 a,x | 4.06 ± 0.55 b,x | 2.91 ± 0.41 a,w |
| Protein Oxidation (nmol hydrazone/mg protein) | |||||
| Day 0 | 4.21 ± 0.88 b,z | 2.24 ± 0.71 a,z | 2.63 ± 0.29 a,z | 2.32 ± 0.51 a,z | 1.98 ± 0.73 a,z |
| 4 | 4.82 ± 0.88 c,z | 2.26 ± 0.78 a,z | 3.39 ± 0.14 b,z | 2.79 ± 0.39 a,b,z | 3.42 ± 0.25 b,y |
| 8 | 8.86 ± 1.63 b,c,y | 10.58 ± 0.34 d,x | 9.55 ± 0.30 c,d,y | 7.21 ± 1.15 a,y | 8.02 ± 0.95 a,b,x |
| 12 | 9.04 ± 2.57 a,b,y | 9.32 ± 1.33 a,b,y | 11.53 ± 1.46 b,x | 7.01 ± 1.30 a,y | 8.27 ± 1.73 a,x |
| Compound | Day 0 | Day 12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Lva | Lvb | Lpa | Lpb | C | Lva | Lvb | Lpa | Lpb | |
| Hexanal | 146.9 ± 54.3 a | 227.4 ± 57.4 a,b | 323.6 ± 58.7 b | 198.6 ± 28.2 a | 218.2 ± 33.6 a,b | 247.3 ± 9.0 b | 162.3 ± 12.8 a | 248.4 ± 30.2 b | 229.6 ± 43.7 b | 279.0 ± 44.3 b |
| Octanal | 26.9 ± 9.0 b | 11.2 ± 4.3 a,z | 21.5 ± 4.3 a,b | 14.5 ± 2.1 a,b | 15.2 ± 2.6 a,b,z | 44.2 ± 3.0 c | 21.3 ± 4.6 a,b,y | 25.6 ± 0.8 b | 15.6 ± 6.3 a | 27.5 ± 0.5 b,y |
| 2-Heptenal | 36.2 ± 11.3 a | 50.3 ± 1.6 a | 118.3 ± 27.6 b | 42.9 ± 8.2 a | 61.6 ± 13.8 a | 42.3 ± 2.3 a | 47.2 ± 1.5 a | 65.8 ± 1.1 c | 55.8 ± 7.2 b | 66.3 ± 2.8 c |
| Nonanal | 51.2 ± 8.8 b,c,z | 37.5 ± 2.3 a,b,z | 59.5 ± 7.2 c | 34.0 ± 7.9 a,z | 38.1 ± 2.0 a,b,z | 87.2 ± 8.6 b,y | 53.9 ± 6.4 a,y | 74.3 ± 0.5 a,b | 73.0 ± 12.8 a,b,y | 74.0 ± 11.7 a,b,y |
| (E)-2-Octenal | 26.2 ± 2.4 a,z | 21.9 ± 7.0 a,z | 63.1 ± 13.3 b | 26.9 ± 5.2 a,y | 34.7 ± 4.8 a,z | 50.4 ± 3.0 y | 41.2 ± 3.2 y | 62.9 ± 13.9 | 59.7 ± 13.8 z | 64.0 ± 4.9 y |
| (E)-2-Nonenal | 23.3 ± 7.2 a | 15.9 ± 3.1 a | 49.0 ± 8.5 b | 18.6 ± 3.7 a | 28.5 ± 4.8 a | 25.5 ± 0.6 a,b | 17.7 ± 2.0 a | 32.0 ± 3.9 b,c | 20.7 ± 9.6 a,b | 38.9 ± 5.6 c |
| 2,4-Nonadienal | 13.1 ± 4.9 a | 12.4 ± 3.0 a | 39.2 ± 7.3 b | 13.2 ± 3.3 a | 23.0 ± 4.2 a | 16.7 ± 0.6 a,b | 14.2 ± 2.1 a | 24.5 ± 6.2 b | 12.3 ± 3.0 a | 32.6 ± 6.7 c |
| (E, E)-2,4-Decadienal | 5.4 ± 2.9 a | 5.2 ± 1.3 a | 16.6 ± 2.1 b,y | 5.9 ± 2.6 a | 8.6 ± 1.8 a,z | 6.4 ± 2.3 a | 5.8 ± 0.8 a | 9.2 ± 2.5 a,z | 7.6 ± 1.5 a | 12.7 ± 1.8 b,y |
| (E, Z)-2,4-Decadienal | 8.2 ± 1.0 a,z | 13.4 ± 2.5 a,b | 40.9 ± 8.3 c | 13.9 ± 3.8 a,b | 22.7 ± 5.0 b,z | 20.1 ± 5.2 a,y | 18.1 ± 4.3 a | 29.3 ± 8.7 a | 21.8 ± 4.0 a | 47.3 ± 0.8 b,y |
| 1-Octen-3-one | 3.2 ± 1.1 a | 4.0 ± 1.5 a | 10.6 ± 1.8 b | 3.6 ± 0.8 a,z | 5.3 ± 1.4 a | 5.8 ± 1.6 | 5.1 ± 0.4 | 7.6 ± 0.3 | 6.3 ± 1.0 y | 7.7 ± 1.5 |
| 1-Pentanol | 2.4 ± 0.6 a,z | 3.3 ± 1.4 a,b,z | 5.4 ± 1.4 b,z | 3.4 ± 0.3 a,b,z | 3.1 ± 0.4 a,b,z | 13.6 ± 1.7 b,y | 7.0 ± 1.5 a,y | 9.5 ± 0.3 a,y | 9.1 ± 1.9 a,y | 10.2 ± 2.3 a,y |
| 4-Heptanol | 1.8 ± 0.3 z | 8.0 ± 3.9 | 5.6 ± 1.0 y | 6.5 ± 4.0 | 2.6 ± 0.5 | 5.2 ± 0.5 b,y | 3.3 ± 0.4 a | 2.9 ± 0.5 a,z | 2.3 ± 1.0 a | 2.4 ± 0.3 a |
| 1-Hexanol | 1.1 ± 0.4 a,z | 2.7 ± 2.0 a,b,z | 4.9 ± 0.6 b,z | 2.9 ± 0.3 a,b,z | 4.7 ± 1.3 b,z | 131.5 ± 21.7 y | 94.0 ± 12.2 y | 105.8 ± 16.6 y | 111.7 ± 26.1 y | 129.6 ± 21.4 y |
| 1-Octen-3-ol | 24.3 ± 3.8 a,z | 33.4 ± 2.5 a,z | 53.6 ± 12.4 b | 29.0 ± 5.3 a,z | 28.4 ± 5.2 a,z | 60.8 ± 6.9 y | 62.4 ± 6.8 y | 70.8 ± 1.0 | 74.8 ± 12.8 y | 74.2 ± 16.2 y |
| 2-Ethyl-1-hexanol | 4.7 ± 0.3 a,z | 29.3 ± 7.4 b | 32.5 ± 17.0 b | 15.9 ± 4.7 a,b | 12.8 ± 2.5 a,b | 52.2 ± 4.7 c,y | 24.9 ± 0.5 b | 22.2 ± 5.3 a,b | 20.9 ± 0.7 a,b | 14.6 ± 3.0 a |
| 1-Octanol | 11.6 ± 3.9 a,z | 9.7 ± 2.7 a,z | 17.3 ± 3.2 b,z | 8.8 ± 1.4 a,z | 10.0 ± 1.4 a,z | 36.6 ± 3.5 c,y | 23.5 ± 3.2 a,b,y | 28.1 ± 0.1 b,y | 17.9 ± 4.0 a,y | 23.4 ± 3.1 a,b,y |
| (E)-2-Octen-1-ol | nd z | nd z | nd z | nd z | nd z | 23.8 ± 3.9 y | 18.2 ± 2.0 y | 21.2 ± 5.8 y | 18.4 ± 5.4 y | 22.4 ± 5.1 y |
| 1-Nonanol | nd z | nd z | nd z | nd z | nd z | 8.7 ± 1.0 y | 8.3 ± 2.2 y | 9.8 ± 2.3 y | 7.3 ± 2.1 y | 11.2 ± 0.5 y |
| Pentanoic acid | 0.2 ± 0.1 a | 0.5 ± 0.2 a,z | 1.4 ± 0.1 c,z | 0.5 ± 0.1 a,z | 1.0 ± 0.2 b,z | 1.7 ± 1.1 | 2.4 ± 1.0 y | 2.8 ± 0.2 y | 3.5 ± 1.2 y | 2.5 ± 0.3 y |
| Hexanoic acid | 6.8 ± 2.6 a,z | 18.4 ± 4.4 a,z | 64.0 ± 10.5 b,z | 26.1 ± 5.2 a,z | 56.0 ± 24.9 b | 59.8 ± 12.4 a,y | 81.5 ± 6.2 a,y | 100.0 ± 1.12 a,b,y | 125.5±34.5 a,b,y | 76.6 ± 8.5 a |
| Octanoic acid | 8.0 ± 1.6 a,z | 197.9 ± 54.2 a | 952.1 ± 96.1 b,y | 223.1 ± 39.9 a,z | 824.4 ± 267.3 b | 16.1 ± 1.5 a,y | 250.6 ± 18.0 b | 581.9 ± 73.8 c,z | 323.0 ± 37.9 b,y | 762.3 ± 34.7 d |
| Decanoic acid | 7.7 ± 1.3 a,z | 316.5 ± 50.4 b,z | 1510.9 ± 236.1 d,y | 327.3 ± 41.2 b,z | 848.4 ± 101.2 c,z | 18.2 ± 3.8 a,y | 515.5 ± 82.7 b,y | 979.6 ± 158.7 c,z | 557.9 ± 42.0 b,y | 1270.2 ± 188.5 d,y |
| Ethyl hexanoate | 6.5 ± 2.5 a,z | 34.9 ± 7.7 b,z | 55.8 ± 9.2 c | 28.4 ± 6.1 b,z | 39.8 ± 4.2 b,z | 97.3 ± 18.8 y | 109.8 ± 9.7 y | 124.6 ± 32.1 | 142.0 ± 34.0 y | 93.7 ± 5.5 y |
| Ethyl octanoate | 7.6 ± 2.3 a,z | 276.1 ± 38.6 a | 1290.9 ± 34.0 b,y | 328.0 ± 57.7 a | 1289.0 ± 392.5 b | 19.8 ± 2.6 a,y | 249.4 ± 52.9 b | 430.6 ± 78.9 c,z | 324.0 ± 3.0 b,c | 626.0 ± 122.5 d |
| Methyl decanoate | nd a | 3.9 ± 1.2 a | 14.7 ± 0.9 b,z | 3.8 ± 0.7 a | 12.5 ± 4.2 b | nd a | 2.7 ± 0.9 b | 5.4 ± 1.3 c,y | 2.1 ± 0.8 b | 8.1 ± 1.2 d |
| Hexyl hexanoate | nd z | nd z | nd z | nd z | nd z | 4.7 ± 1.5 y | 2.9 ± 0.9 y | 4.2 ± 1.8 y | 4.2 ± 2.0 y | 4.2 ± 1.1 y |
| Ethyl decanoate | 4.6 ± 2.6 a,z | 3161.4 ± 576.0 a,y | 13,321.0 ± 1281.4 b,y | 3201.1 ± 478.0 a,y | 11,788.6 ± 4044.9 b | 42.5 ± 2.4 a,y | 1190.7 ± 266.9 b,z | 2884.3 ± 1011.4 c,z | 1546.1 ± 271.9 b,z | 5680.9 ± 430.1 d |
| 3-Methylbutyl octanoate | nd a | 35.3 ± 6.3 b,y | 160.4 ± 23.0 d,z | 36.7 ± 4.4 b | 102.6 ± 15.9 c | nd a | 17.2 ± 4.4 a,b,z | 91.6 ± 22.5 c,y | 31.5 ± 3.1 b | 104.4 ± 4.1 c |
| Propyl decanoate | nd a | 2.9 ± 0.2 a | 12.6 ± 2.3 b,y | 2.8 ± 0.1 a | 9.9 ± 3.3 b | nd a | 2.6 ± 1.1 b | 5.5 ± 1.2 c,z | 2.9 ± 0.6 b | 9.2 ± 0.7 d |
| Butyl decanoate | nd a | 2.7 ± 0.5 a | 12.2 ± 2.4 b,y | 2.7 ± 0.3 a | 9.1 ± 3.5 b | nd a | 2.3 ± 0.9 b | 4.9 ± 1.4 c,z | 2.6 ± 0.7 b | 9.6 ± 0.6 d |
| Methyl dodecanoate | nd a | 0.3 ± 0.0 a,z | 2.9 ± 0.4 b,y | 0.4 ± 0.1 a,z | 2.7 ± 0.8 b | nd a | 1.2 ± 0.1 b,y | 1.4 ± 0.5 b,z | 1.0 ± 0.3 b,y | 2.6 ± 0.4 c |
| 3-Methylbutyl decanoate | nd a | 40.1 ± 7.6 b | 182.2 ± 35.2 d,y | 37.5 ± 5.4 b | 112.0 ± 15.1 c | nd a | 36.3 ± 11.5 b | 81.1 ± 18.8 c,z | 44.8 ± 3.5 b | 137.2 ± 35.7 d |
| p-Cymene | 0.3 ± 0.2 y | 2.2 ± 1.9 | 1.4 ± 0.2 y | 0.8 ± 0.1 y | 1.2 ± 0.4 | 0.8 ± 0.1 a,z | 0.8 ± 0.0 a | 1.2 ± 0.1 c,z | 0.9 ± 0.1 a,z | 1.1 ± 0.0 b |
| Trimethyl benzene | 0.4 ± 0.1 a,y | 0.9 ± 0.3 b,c,y | 1.2 ± 0.1 c,y | 0.7 ± 0.1 a,b,y | 1.0 ± 0.1 b,c | 0.4 ± 0.1 a,z | 0.7 ± 0.0 b,z | 0.9 ± 0.1 c,z | 0.7 ± 0.1 b,z | 1.1 ± 0.1 d |
| Benzaldehyde | 18.3 ± 5.6 a,z | 17.4 ± 4.7 a,z | 40.5 ± 6.7 b,z | 17.5 ± 3.3 a,z | 27.5 ± 6.6 a,z | 64.1 ± 1.5 a,y | 93.9 ± 19.5 a,b,y | 75.5 ± 8.1 a,y | 114.9 ± 15.9 b,y | 93.7 ± 6.0 a,b,y |
| Phenylethyl alcohol | 1.2 ± 0.7 a,z | 29.0 ± 2.1 b | 145.3 ± 18.1 d,y | 39.4 ± 6.7 b | 105.9 ± 12.0 c | 30.2 ± 3.7 a,y | 41.0 ± 6.1 a | 92.0 ± 2.2 b,z | 51.3 ± 7.1 a | 119.3 ± 20.2 c |
| Carbon disulfide | 0.6 ± 0.1 a | 1.3 ± 0.4 b | 1.0 ± 0.2 a,b | 0.5 ± 0.1 a | 1.0 ± 0.3 a,b | 1.4 ± 0.5 b | 0.7 ± 0.3 a,b | 0.7 ± 0.2 a,b | 0.5 ± 0.2 a | 1.0 ± 0.4 a,b |
| 5-Methyl thiazole | nd z | nd z | nd z | nd z | nd z | 11.3 ± 4.0 a,y | 9.7 ± 0.8 a,z | 38.4 ± 16.7 b,y | 8.9 ± 2.7 a,y | 20.0 ± 9.7 a,y |
| 2-Pentyl furan | 12.1 ± 3.1 a,z | 19.7 ± 4.5 a,z | 29.5 ± 6.4 b | 12.9 ± 1.7 a,z | 14.8 ± 1.2 a,z | 23.6 ± 3.9 y | 26.7 ± 3.0 y | 38.4 ± 6.7 | 37.6 ± 4.9 y | 36.7 ± 8.0 y |
| Total concentrations of the main groups of compounds * | ||||||||||
| ∑ Aldehydes | 422.7 ± 91.3 a,z | 450.0 ± 53.7 a | 853.0 ± 155.9 b | 416.2 ± 65.4 a | 520.5 ± 75.9 a,z | 685.9 ± 31.5 b,c,y | 488.3 ± 32.6 a | 660.0 ± 75.9 b,c | 570.4 ± 100.6 a,b | 758.4 ± 74.4 c,y |
| ∑ Ketones | 30.9 ± 7.3 | 27.5 ± 7.0 z | 37.1 ± 3.0 z | 23.4 ± 3.2 z | 27.1 ± 5.9 z | 38.2 ± 6.3 a | 43.0 ± 1.6 a,y | 58.3 ± 6.3 b,y | 55.7 ± 6.3 b,y | 47.1 ± 5.6 a,b,y |
| ∑ Alcohols | 66.5 ± 13.1 a,z | 107.1 ± 13.8 a,z | 142.4 ± 35.1 b,z | 84.3 ± 12.3 a,z | 79.2 ± 8.9 a,z | 379.8 ± 48.4 b,y | 267.6 ± 28.8 a,y | 305.9 ± 18.8 a,b,y | 301.0 ± 42.9 a,b,y | 323.7 ± 42.6 a,b,y |
| ∑ Hydrocarbons | 26.0 ± 9.8 a | 26.4 ± 8.5 a,z | 48.5 ± 11.7 b | 25.5 ± 4.8 a,z | 25.5 ± 2.2 a,z | 36.1 ± 3.8 a | 40.6 ± 1.5 a,b,y | 52.6 ± 8.1 b | 54.8 ± 10.5 b,y | 45.2 ± 4.3 a,b,y |
| ∑ Acids | 37.5 ± 9.0 a,z | 571.0 ± 102.1 b,z | 2765.7 ± 384.0 d,y | 627.7 ± 92.4 b,z | 1844.0 ± 340.9 c | 138.8 ± 14.3 a,y | 925.8 ± 123.0 b,y | 1828.2 ± 250.1 c,z | 1092.1 ± 124.4 b,y | 2278.9 ± 227.5 d |
| ∑ Esters | 18.9 ± 6.5 a,z | 4289.2 ± 753.4 a,y | 18,192.2 ± 1860.4 b,y | 4468.6 ± 664.5 a,y | 16,072.1 ± 5197.0 b | 196.9 ± 27.6 a,y | 2140.8 ± 276.4 b,z | 4885.7 ± 1280.0 c,z | 3020.2 ± 401.0 b,z | 8856.2 ± 547.3 d |
| ∑ Benzenic compounds | 25.5 ± 7.6 a,z | 57.3 ± 10.0 b,z | 210.5 ± 28.3 d | 65.3 ± 11.4 b,z | 141.7 ± 15.4 c,z | 126.0 ± 7.1 a,y | 158.9 ± 27.6 a,b,y | 203.3 ± 11.5 b | 192.2 ± 25.4 b,y | 251.7 ± 21.1 c,y |
| ∑ Sulfur compounds | 0.6 ± 0.1 a,z | 1.3 ± 0.4 b,z | 1.0 ± 0.2 a,b,z | 0.5 ± 0.1 a,z | 1.0 ± 0.3 a,b | 12.7 ± 3.8 a,y | 10.3 ± 1.1 a,y | 39.1 ± 16.6 b,y | 9.4 ± 2.9 a,y | 21.1 ± 9.6 a |
| ∑ Furanic compounds | 14.1 ± 3.0 a,z | 23.1 ± 6.2 a | 34.7 ± 7.9 b | 15.2 ± 2.5 a,z | 19.7 ± 0.9 a | 26.2 ± 4.1 a,y | 30.3 ± 3.1 a,b | 43.6 ± 7.0 b | 40.6 ± 5.1 a,b,y | 40.5 ± 9.0 a,b |
| Principal Component | Compounds | Loadings |
|---|---|---|
| PC 1 | Butyl decanoate | 0.981 |
| Propyl decanoate | 0.967 | |
| 3-Methylbutyl decanoate | 0.967 | |
| Octanoic acid | 0.964 | |
| Phenylethyl alcohol | 0.956 | |
| 3-Methylbutyl octanoate | 0.955 | |
| Methyl dodecanoate | 0.949 | |
| Methyl decanoate | 0.945 | |
| PC 2 | Benzaldehyde | 0.956 |
| 1-Hexanol | 0.942 | |
| 1-Nonanol | 0.919 | |
| Ethyl-hexanoate | 0.915 | |
| Pentanoic acid | 0.909 | |
| (E)-2-octen-1-ol | 0.908 | |
| 1-Octen-3-ol | 0.906 | |
| Hexyl hexanoate | 0.904 | |
| Hexanoic acid | 0.899 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcón, M.; López-Viñas, M.; Pérez-Coello, M.S.; Díaz-Maroto, M.C.; Alañón, M.E.; Soriano, A. Effect of Wine Lees as Alternative Antioxidants on Physicochemical and Sensorial Composition of Deer Burgers Stored during Chilled Storage. Antioxidants 2020, 9, 687. https://doi.org/10.3390/antiox9080687
Alarcón M, López-Viñas M, Pérez-Coello MS, Díaz-Maroto MC, Alañón ME, Soriano A. Effect of Wine Lees as Alternative Antioxidants on Physicochemical and Sensorial Composition of Deer Burgers Stored during Chilled Storage. Antioxidants. 2020; 9(8):687. https://doi.org/10.3390/antiox9080687
Chicago/Turabian StyleAlarcón, Marina, Manuel López-Viñas, María Soledad Pérez-Coello, María Consuelo Díaz-Maroto, María Elena Alañón, and Almudena Soriano. 2020. "Effect of Wine Lees as Alternative Antioxidants on Physicochemical and Sensorial Composition of Deer Burgers Stored during Chilled Storage" Antioxidants 9, no. 8: 687. https://doi.org/10.3390/antiox9080687
APA StyleAlarcón, M., López-Viñas, M., Pérez-Coello, M. S., Díaz-Maroto, M. C., Alañón, M. E., & Soriano, A. (2020). Effect of Wine Lees as Alternative Antioxidants on Physicochemical and Sensorial Composition of Deer Burgers Stored during Chilled Storage. Antioxidants, 9(8), 687. https://doi.org/10.3390/antiox9080687