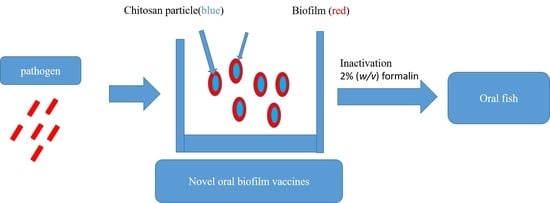
Protective Efficacy of Novel Oral Biofilm Vaccines against Photobacterium damselae subsp. damselae Infection in Giant Grouper, Epinephelus lanceolatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Fish Maintenance
2.2.1. Bacterial Strains
2.2.2. Cultured and Quantified Photobacterium damselae subsp. damselae Biofilm
2.2.3. Preparation of Feed-Based Photobacterium damselae subsp. damselae Biofilm Vaccine (PBV), Photobacterium damselae subsp. damselae Whole-Cell Vaccine (PWV), and Chitosan Particle (CP)
2.3. Vaccination
2.4. Phagocytosis Analyses
2.5. Albumin–Globulin Ratio Analyses
2.6. Serum Lysozyme Assay
2.7. IgM Assay
2.8. Immune-Related Genes Expression by qRT-PCR
2.9. Challenge with Photobacterium damselae subsp. damselae
2.10. Statistical Analysis
3. Results
3.1. Scanning Electron Microscopy(SEM) Observation of Photobacterium damselae subsp. damselae Biofilm
3.2. Innate Immune Response after Vaccination
3.3. Antibody Production
3.4. Immune-Related Gene Analysis
3.5. Relative Percentage Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadovy, Y.; Donaldson, T.; Graham, T.; McGilvray, F.; Muldoon, G.; Phillips, M.; Rimmer, M.; Smith, A.; Yeeting, B. While Stocks Last: The Live Reef Food Fish Trade; Asian Development Bank: Mandaluyong, Philippines, 2003. [Google Scholar]
- Tao, Z.; Shen, C.; Zhou, S.-M.; Yang, N.; Wang, G.-L.; Wang, Y.-J.; Xu, S.-L. An outbreak of Photobacterium damselae subsp. damselae infection in cultured silver pomfret Pampus argenteus in eastern china. Aquaculture 2018, 492, 201–205. [Google Scholar] [CrossRef]
- Sharma, S.; Pradeep, M.; Sadhu, N.; Dube, P.; Vijayan, K.K. First report of isolation and characterization of Photobacterium damselae subsp. damselae from cage-farmed cobia (Rachycentron canadum). J. Fish Dis. 2017, 40, 953–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, G.; Lafay, B.; Ruimy, R.; Breittmayer, V.; Nicolas, J.L.; Christen, R. Small-subunit rrna sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (snieszko et al.) janssen and surgalla to the genus Photobacterium as Photobacterium damsela subsp. Piscicida comb. Int. J. Syst. Bacteriol. 1995, 45, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.K.; Sutton, D.C.; Fuerst, J.A.; Reichelt, J.L. Evaluation of the genus Listonella and reassignment of Listonella damsela (love et al.) macdonell and colwell to the genus Photobacterium as Photobacterium damsela comb. Nov. with an emended description. Int. J. Syst. Bacteriol. 1991, 41, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.; Wilson, R.; Hollis, D.; Weaver, R.; Miller, H.; Tacket, C.; Hickman, F.; Blake, P. Illness caused by Vibrio damsela and Vibrio hollisae. Lancet 1982, 319, 1294–1297. [Google Scholar] [CrossRef]
- Love, M.; Teebken-Fisher, D.; Hose, J.E.; Farmer, J.J.; Hickman, F.W.; Fanning, G.R. Vibrio damsela, a marine bacterium, causes skin ulcers on the damselfish chromis punctipinnis. Science 1981, 214, 1139–1140. [Google Scholar] [CrossRef]
- Chiu, T.H.; Kao, L.Y.; Chen, M.L. Antibiotic resistance and molecular typing of Photobacterium damselae subsp. damselae, isolated from seafood. J. Appl. Microbiol. 2013, 114, 1184–1192. [Google Scholar] [CrossRef]
- Vinitnantharat, S.; Gravningen, K.; Greger, E. Fish vaccines. Adv. Vet. Med. 1999, 41, 539–550. [Google Scholar]
- Forchielli, M.L.; Walker, W.A. The role of gut-associated lymphoid tissues and mucosal defence. Br. J. Nutr. 2005, 93, S41–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, I.; Shankar, K.; Mohan, C.; Kalita, B. Uptake and processing of biofilm and free-cell vaccines of Aeromonas hydrophila in indian major carps and common carp following oral vaccination antigen localization by a monoclonal antibody. Dis. Aquat. Org. 2000, 43, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Vinay, T.; Girisha, S.; D’souza, R.; Jung, M.-H.; Choudhury, T.; Patil, S.S. Bacterial biofilms as oral vaccine candidates in aquaculture. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 2016, 37, 57–62. [Google Scholar] [CrossRef]
- Rombout, J.; Kiron, V. Mucosal vaccination of fish. Cell Biol. Immunol. 2014, 383, 56–67. [Google Scholar]
- Mutoloki, S.; Munang’andu, H.M.; Evensen, Ø. Oral vaccination of fish–antigen preparations, uptake, and immune induction. Front. Immunol. 2015, 6, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, D.; Asha, A.; Shankar, K.; Mohan, C. Evaluation of biofilm of Aeromonas hydrophila for oral vaccination of Clarias batrachus—A carnivore model. Fish Shellfish Immunol. 2004, 16, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.K.; Kumar, B.N.; Poojary, S.R.; Abhiman, P.; Patil, P.; Ramesh, K.; Shankar, K. Evaluation of biofilm of vibrio anguillarum for oral vaccination of asian seabass, Lates calcarifer (bloch, 1790). Fish Shellfish Immunol. 2019, 94, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kumar, B.N.; Tyagi, A.; Holeyappa, S.A.; Singh, N.K. Identification of novel vaccine candidates in the whole-cell Aeromonas hydrophila biofilm vaccine through reverse vaccinology approach. Fish Shellfish Immunol. 2021, 114, 132–141. [Google Scholar] [CrossRef]
- Kahieshesfandiari, M.; Sabri, M.Y.; Ina-Salwany, M.Y.; Hassan, M.D.; Noraini, O.; Ajadi, A.A.; Isiaku, A.I. Streptococcosis in Oreochromis sp.: Is feed-based biofilm vaccine of Streptococcus agalactiae effective? Aquac. Int. 2019, 27, 817–832. [Google Scholar] [CrossRef]
- Azad, I.; Shankar, K.; And, C.M.; Kalita, B. Biofilm vaccine of Aeromonas hydrophila–standardization of dose and duration for oral vaccination of carps. Fish Shellfish Immunol. 1999, 9, 519–528. [Google Scholar] [CrossRef]
- Su, F.-J.; Chen, M.-M. Protective efficacy of novel oral biofilm vaccines against Lactococcus garvieae infection in mullet, Mugil cephalus. Vaccines 2021, 9, 844. [Google Scholar] [CrossRef]
- Osorio, C.R.; Toranzo, A.E.; Romalde, J.L.; Barja, J.L. Multiplex pcr assay for urec and 16s rrna genes clearly discriminates between both subspecies of Photobacterium damselae. Dis. Aquat. Org. 2000, 40, 177–183. [Google Scholar] [CrossRef]
- Tote, K.; Berghe, D.V.; Maes, L.; Cos, P. A new colorimetric microtitre model for the detection of Staphylococcus aureus biofilms. Lett. Appl. Microbiol. 2008, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-W.; Tu, C.-Y.; Wang, W.-S. In vivo effects of adding singular or combined anti-oxidative vitamins and/or minerals to diets on the immune system of tilapia (Oreochromis hybrids) peripheral blood monocyte-derived, anterior kidney-derived, and spleen-derived macrophages. Veter-Immunol. Immunopathol. 2007, 115, 87–99. [Google Scholar]
- Thanga Viji, V.; Deepa, K.; Velmurugan, S.; Donio, M.B.; Adlin Jenifer, J.; Babu, M.M.; Citarasu, T. Vaccination strategies to protect goldfish Carassius auratus against Aeromonas hydrophila infection. Dis. Aquat. Org. 2013, 104, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.-H.; Choi, S. technology. Effects of prunella vulgaris labiatae extract on specific and non-specific immune responses in tilapia (Oreochromis niloticus). J. Anim. Sci. Technol. 2014, 56, 3. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Tan, X.; Ye, H.; Zou, C.; Ye, C.; Wang, A. Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 73, 234–244. [Google Scholar] [CrossRef]
- Lam, F.W.-S.; Wu, S.-Y.; Lin, S.-J.; Lin, C.-C.; Chen, Y.-M.; Wang, H.-C.; Chen, T.-Y.; Lin, H.-T.; Lin, J.H.-Y. The expression of two novel orange-spotted grouper (Epinephelus coioides) tnf genes in peripheral blood leukocytes, various organs, and fish larvae. Fish Shellfish Immunol. 2011, 30, 618–629. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Y.; Wei, Z.; Mai, H.; Liu, Q.; Liu, B.; Zhuang, Y.; Zou, D.; Zhang, W.; Liu, X.; et al. The effects of dietary compound plant extracts on growth performance, liver and intestine health, and immune related genes expression in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Fish Shellfish Immunol. 2021, 119, 11–18. [Google Scholar] [CrossRef]
- King, R.K.; Flick, G.J., Jr.; Pierson, D.; Smith, S.A.; Boardman, G.D.; Coale, C.W., Jr. Identification of bacterial pathogens in biofilms of recirculating aquaculture systems. J. Aquat. Food Prod. Technol. 2004, 13, 125–133. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Labella, A.; Sanchez-Montes, N.; Berbel, C.; Aparicio, M.; Castro, D.; Manchado, M.; Borrego, J.J. Toxicity of Photobacterium damselae subsp. damselae strains isolated from new cultured marine fish. Dis. Aquat. Org. 2010, 92, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loera-Muro, A.; Guerrero-Barrera, A.; Tremblay DN, Y.; Hathroubi, S.; Angulo, C. Bacterial biofilm-derived antigens: A new strategy for vaccine development against infectious diseases. Expert Rev. Vaccines 2021, 20, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Kaattari, S.L.; Christensen, J.M. Effectiveness of an oral enteric coated vibrio vaccine for use in salmonid fish. Immunol. Investig. 1992, 21, 353–364. [Google Scholar] [CrossRef]
- Jenkins, P.; Wrathmell, A.; Harris, J.; Pulsford, A. The effects of different adjuvants on intestinal antigen absorption and subsequent immune responses of the tilapian Oreochromis mossambicus. Fish Shellfish Immunol. 1994, 4, 167–177. [Google Scholar] [CrossRef]
- Davidson, G.; Ellis, A.; Secombes, C. Route of immunization influences the generation of antibody secreting cells in the gut of rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 1993, 17, 373–376. [Google Scholar] [CrossRef]
- Firdaus-Nawi, M.; Yusoff, S.M.; Yusof, H.; Abdullah, S.Z.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2013, 45, 87–96. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, R.; Prasad, K.P.; Vani, T.; Kumar, R. Effect of dietary chitosan on haematology, innate immunity and disease resistance of asian seabass Lates calcarifer (bloch). Aquac. Res. 2014, 45, 983–993. [Google Scholar] [CrossRef]
- Kumar, S.; Raman, R.P.; Kumar, K.; Pandey, P.K.; Kumar, N.; Mallesh, B.; Mohanty, S.; Kumar, A. Effect of azadirachtin on haematological and biochemical parameters of argulus-infested goldfish Carassius auratus (linn. 1758). Fish Physiol. Biochem. 2013, 39, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Wiegertjes, G.; Stet, R.; Parmentier, H.K.; van Muiswinkel, W.B. Immunogenetics of disease resistance in fish: A comparative approach. Dev. Comp. Immunol. 1996, 20, 365–381. [Google Scholar] [CrossRef]
- Ji, R.; Zou, W.; Hu, S.; Yan, Q. Vaccination in three different ways against vibriosis of seriola dumerili caused by Vibrio hollisae. Chin. J. Oceanol. Limnol. 2008, 26, 233–237. [Google Scholar] [CrossRef]
- Siriyappagouder, P.; Shankar, K.; Kumar, B.N.; Patil, R.; Byadgi, O.V. Evaluation of biofilm of Aeromonas hydrophila for oral vaccination of Channa striatus. Fish Shellfish Immunol. 2014, 41, 581–585. [Google Scholar] [CrossRef]
- Mamun, M.; Nasren, S.; Abhiman, P.; Rathore, S.; Sowndarya, N.; Ramesh, K.; Shankar, K.M. Investigation of production, formation and characterization of biofilm cells of Aeromonas hydrophila for oral vaccination of fish. J. Exp. Zool. India 2019, 22, 1115–1123. [Google Scholar]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Pei, L.; Wang, P.; Liu, L.; Li, G.; Liu, B.; Lǚ, Z.; Hiromasa, T.; Pan, H.; Ogura, A. Molecular characterization and evolution analysis of two forms of tlr5 and tlr13 genes base on Larimichthys crocea genome data. J. Genom. 2020, 2020, 4895037. [Google Scholar] [CrossRef]
- Liu, Y.; Mo, C.-F.; Luo, X.-Y.; Li, H.; Guo, H.-J.; Sun, H.; Hu, S.; Li, L.-M.; Wang, Y.-T.; Yang, S.-X.; et al. Activation of toll-like receptor 3 induces interleukin-1 receptor antagonist expression by activating the interferon regulatory factor 3. J. Innate Immun. 2020, 12, 304–320. [Google Scholar] [CrossRef]






| Name | Sequence | Reference |
|---|---|---|
| IL-1β-F | CGACATGGTGCGGTTTCTCT | [27] |
| IL-1β-R | CTCTGCTGTGCTGATTACCAGTT | |
| TNF-α-F | GCTGCGGCTCGAAGACAAT | [27] |
| TNF-α-R | CAGACGGTGCGGATGGAGT | |
| TLR 3-F | TCTCCATTCCGTCACCTTCC | [26] |
| TLR 3-R | TCATCCAGCCCGTTACTATCC | |
| MHC-II-F | CCACCCGAACAAACAGACC | [26] |
| MHC-II-R | TGATGCCCCCTCCAACACT | |
| IL-8-F | AGTCATTGTCATCTCCATTGCG | [28] |
| IL-8-R | AAACTTCTTGGCCTGTCCTTTT | |
| β-actin-F | TACGAGCTGCCTGACGGACA | [26] |
| β-actin-R | GGCTGTGATCTCCTTCTGC |
| Group | Final Mortality (%) | RPS (%) | |
|---|---|---|---|
| PBV | n = 25 | 32 | 62 |
| PWV | n = 25 | 84 | 0 |
| CP | n = 25 | 84 | 0 |
| PBS | n = 25 | 84 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, F.-J.; Chen, M.-M. Protective Efficacy of Novel Oral Biofilm Vaccines against Photobacterium damselae subsp. damselae Infection in Giant Grouper, Epinephelus lanceolatus. Vaccines 2022, 10, 207. https://doi.org/10.3390/vaccines10020207
Su F-J, Chen M-M. Protective Efficacy of Novel Oral Biofilm Vaccines against Photobacterium damselae subsp. damselae Infection in Giant Grouper, Epinephelus lanceolatus. Vaccines. 2022; 10(2):207. https://doi.org/10.3390/vaccines10020207
Chicago/Turabian StyleSu, Feng-Jie, and Meei-Mei Chen. 2022. "Protective Efficacy of Novel Oral Biofilm Vaccines against Photobacterium damselae subsp. damselae Infection in Giant Grouper, Epinephelus lanceolatus" Vaccines 10, no. 2: 207. https://doi.org/10.3390/vaccines10020207
APA StyleSu, F. -J., & Chen, M. -M. (2022). Protective Efficacy of Novel Oral Biofilm Vaccines against Photobacterium damselae subsp. damselae Infection in Giant Grouper, Epinephelus lanceolatus. Vaccines, 10(2), 207. https://doi.org/10.3390/vaccines10020207