The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation
Abstract
:1. Introduction
2. Strategies to Provide Evidence-Based Information on COVID-19 Vaccines
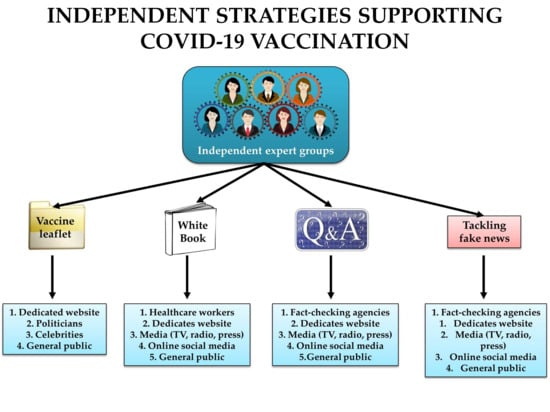
2.1. Organizing Expert Groups Communicating Science on COVID-19 Vaccines
2.2. Tracking and Tackling Fake News on COVID-19 Vaccines
2.3. Equipping Celebrities and Politicians with Scientific Information on COVID-19 Vaccines
2.4. Supporting the COVID-19 Vaccination through Public Letters and Statements
2.5. No Tolerance to False and Manipulated Claims on COVID-19 Vaccines
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burki, T. Outbreak of coronavirus disease 2019. Lancet Infect. Dis. 2020, 20, 292–293. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta BioMed. Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Lenzen, M.; Li, M.; Malik, A.; Pomponi, F.; Sun, Y.-Y.; Wiedmann, T.; Faturay, F.; Fry, J.; Gallego, B.; Geschke, A.; et al. Global socio-economic losses and environmental gains from the Coronavirus pandemic. PLoS ONE 2020, 15, e0235654. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.F.; Becker, A.D.; Grenfell, B.T.; Metcalf, C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat. Med. 2020, 26, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Petzold, M.B.; Bendau, A.; Plag, J.; Pyrkosch, L.; Mascarell Maricic, L.; Betzler, F.; Rogoll, J.; Große, J.; Ströhle, A. Risk, resilience, psychological distress, and anxiety at the beginning of the COVID-19 pandemic in Germany. Brain Behavior 2020, 10, e01745. [Google Scholar] [CrossRef] [PubMed]
- Rapanta, C.; Botturi, L.; Goodyear, P.; Guàrdia, L.; Koole, M. Online University Teaching During and After the Covid-19 Crisis: Refocusing Teacher Presence and Learning Activity. Postdigital Sci. Educ. 2020, 2, 923–945. [Google Scholar] [CrossRef]
- Dubey, M.J.; Ghosh, R.; Chatterjee, S.; Biswas, P.; Chatterjee, S.; Dubey, S. COVID-19 and addiction. Diabetes Metab. Syndr. 2020, 14, 817–823. [Google Scholar] [CrossRef]
- Rzymski, P.; Nowicki, M. COVID-19-related prejudice toward Asian medical students: A consequence of SARS-CoV-2 fears in Poland. J. Infect. Public Health 2020, 13, 873–876. [Google Scholar] [CrossRef]
- Nowakowska, J.; Sobocińska, J.; Lewicki, M.; Lemańska, Ż.; Rzymski, P. When science goes viral: The research response during three months of the COVID-19 outbreak. Biomed. Pharmacother. 2020, 129, 110451. [Google Scholar] [CrossRef]
- Gianola, S.; Jesus, T.S.; Bargeri, S.; Castellini, G. Characteristics of academic publications, preprints, and registered clinical trials on the COVID-19 pandemic. PLoS ONE 2020, 15, e0240123. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Nowicki, M.; Mullin, G.E.; Abraham, A.; Rodríguez-Román, E.; Petzold, M.B.; Bendau, A.; Sahu, K.K.; Ather, A.; Naviaux, A.-F.; et al. Quantity does not equal quality: Scientific principles cannot be sacrificed. Int. Immunopharmacol. 2020, 86, 106711. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef] [PubMed]
- Cusinato, J.; Cau, Y.; Calvani, A.M.; Mori, M. Repurposing drugs for the management of COVID-19. Expert Opin. Ther. Patents 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Sternberg, A.; McKee, D.L.; Naujokat, C. Novel Drugs Targeting the SARS-CoV-2/COVID-19 Machinery. Curr. Top. Med. Chem. 2020, 20, 1423–1433. [Google Scholar] [CrossRef]
- Wondmkun, Y.T.; Mohammed, O.A. A Review on Novel Drug Targets and Future Directions for COVID-19 Treatment. Biologics 2020, 14, 77–82. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Sun, M.; Xu, Y.; He, H.; Zhang, L.; Wang, X.; Qiu, Q.; Sun, C.; Guo, Y.; Qiu, S.; Ma, K. A potentially effective treatment for COVID-19: A systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease. Int. J. Infect. Dis. 2020, 98, 334–346. [Google Scholar] [CrossRef]
- Kadkhoda, K. Herd Immunity to COVID-19: Alluring and Elusive. Am. J. Clin. Pathol. 2021. [Google Scholar] [CrossRef]
- Sridhar, D.; Gurdasani, D. Herd immunity by infection is not an option. Science 2021, 371, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Alwan, N.A.; Burgess, R.A.; Ashworth, S.; Beale, R.; Bhadelia, N.; Bogaert, D.; Dowd, J.; Eckerle, I.; Goldman, L.R.; Greenhalgh, T.; et al. Scientific consensus on the COVID-19 pandemic: We need to act now. Lancet 2020, 396, e71–e72. [Google Scholar] [CrossRef]
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol 2020, 892, 173751. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Dis. 2020, 19, 667–668. [Google Scholar] [CrossRef]
- Mullard, A. How COVID vaccines are being divvied up around the world. Nature 2020. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar] [CrossRef]
- Porter, C. In Canada, First Vaccines Leave Health Workers in Tears of Relief. New York Times, 14 December 2020. [Google Scholar]
- Kabamba Nzaji, M.; Kabamba Ngombe, L.; Ngoie Mwamba, G.; Banza Ndala, D.B.; Mbidi Miema, J.; Luhata Lungoyo, C.; Lora Mwimba, B.; Cikomola Mwana Bene, A.; Mukamba Musenga, E. Acceptability of Vaccination Against COVID-19 Among Healthcare Workers in the Democratic Republic of the Congo. Pragmat Obs. Res. 2020, 11, 103–109. [Google Scholar] [CrossRef]
- Gadoth, A.; Halbrook, M.; Martin-Blais, R.; Gray, A.; Tobin, N.H.; Ferbas, K.G.; Aldrovandi, G.M.; Rimoin, A.W. Assessment of COVID-19 vaccine acceptance among healthcare workers in Los Angeles. medRxiv 2020. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Detoc, M.; Bruel, S.; Tardy, B.; Rozaire, O.; Frappe, P.; Botelho-Nevers, E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: A cross sectional survey. J. Hosp. Infect. 2020. [Google Scholar] [CrossRef]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Nkengasong, J.N.; Ndembi, N.; Tshangela, A.; Raji, T. COVID-19 vaccines: How to ensure Africa has access. Nature 2020, 586, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020, 396, 1614–1616. [Google Scholar] [CrossRef]
- Phizackerley, D. Covid-19 vaccination-we need more than the ‘mum test’. Drug Ther. Bull. 2021, 59, 2. [Google Scholar] [CrossRef]
- Johnson, N.F.; Velásquez, N.; Restrepo, N.J.; Leahy, R.; Gabriel, N.; El Oud, S.; Zheng, M.; Manrique, P.; Wuchty, S.; Lupu, Y. The online competition between pro- and anti-vaccination views. Nature 2020, 582, 230–233. [Google Scholar] [CrossRef]
- Van der Linden, S.; Roozenbeek, J.; Compton, J. Inoculating Against Fake News About COVID-19. Front. Psychol. 2020, 11, 566790. [Google Scholar] [CrossRef]
- Gwenzi, W.; Rzymski, P. When silence goes viral, Africa sneezes! A perspective on Africa’s subdued research response to COVID-19 and a call for local scientific evidence. Environ. Res. 2020, 194, 110637. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020, 1–4. [Google Scholar] [CrossRef]
- Lin, C.; Tu, P.; Beitsch, L.M. Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2020, 9, 16. [Google Scholar] [CrossRef]
- Gruber, M.F.; Marshall, V.B. Regulation and Testing of Vaccines. Plotkin’s Vaccines 2018, 1547–1565.e1542. [Google Scholar] [CrossRef]
- Eise, J. What institutions can do to improve science communication. Nature 2019. [Google Scholar] [CrossRef] [PubMed]
- Brownell, S.E.; Price, J.V.; Steinman, L. Science Communication to the General Public: Why We Need to Teach Undergraduate and Graduate Students this Skill as Part of Their Formal Scientific Training. J. Undergrad. Neurosci. Educ. 2013, 12, E6–E10. [Google Scholar] [PubMed]
- Science Against Pandemic Initiative. Polish White Book. Available online: https://naukaprzeciwpandemii.pl/en (accessed on 27 December 2020).
- Diseases, The Lancet Infectious. The COVID-19 infodemic. Lancet Infect. Dis. 2020, 20, 875. [CrossRef]
- Orso, D.; Federici, N.; Copetti, R.; Vetrugno, L.; Bove, T. Infodemic and the spread of fake news in the COVID-19-era. Eur. J. Emerg. Med. Off. J. Eur. Soc. Emerg. Med. 2020, 27, 327–328. [Google Scholar] [CrossRef]
- Islam, M.S.; Sarkar, T.; Khan, S.H.; Mostofa Kamal, A.-H.; Hasan, S.M.M.; Kabir, A.; Yeasmin, D.; Islam, M.A.; Amin Chowdhury, K.I.; Anwar, K.S.; et al. COVID-19–Related Infodemic and Its Impact on Public Health: A Global Social Media Analysis. Am. J. Trop. Med. Hyg. 2020, 103, 1621–1629. [Google Scholar] [CrossRef]
- Brandtzaeg, P.; Følstad, A. Trust and Distrust in Online Fact-Checking Services. Commun. ACM 2017, 60, 65. [Google Scholar] [CrossRef]
- Moraes, M.; Gountas, J.; Gountas, S.; Sharma, P. Celebrity influences on consumer decision making: New insights and research directions. J. Mark. Manag. 2019, 35, 1159–1192. [Google Scholar] [CrossRef]
- Jun-Hwa, C.; Ting, H.; Cham, T.H.; Memon, M.J.I.R. The effect of selfie promotion and celebrity endorsed advertisement on decision-making processes. Internet Res. 2019, 29, 552–577. [Google Scholar]
- Meshi, D.; Biele, G.; Korn, C.W.; Heekeren, H.R. How expert advice influences decision making. PLoS ONE 2012, 7, e49748. [Google Scholar] [CrossRef]
- Klee, A.J. The utilization of expert opinion in decision-making. AIChE J. 1972, 18, 1107–1115. [Google Scholar] [CrossRef]
- Rzymski, P.; Nowicki, M. Preventing COVID-19 prejudice in academia. Science 2020, 367, 1313. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.; Carroll, D.; Colwell, R.; Corley, R.B.; Daszak, P.; Drosten, C.; Enjuanes, L.; Farrar, J.; Field, H.; Golding, J.; et al. Statement in support of the scientists, public health professionals, and medical professionals of China combatting COVID-19. Lancet 2020, 395, e42–e43. [Google Scholar] [CrossRef]
- Butler, T.; Paterson, K.; Stanford, D.; Moore, S.; West, E.; Kausar, N.; Aslett-Bentley, A.; Convery, L. Joint BACPR/BDA/PHNSG statement on nutrition and cardiovascular health post-COVID-19 pandemic. Br. J. Cardiol. 2020, 27, 79. [Google Scholar]
- Burki, T. The online anti-vaccine movement in the age of COVID-19. Lancet Digit. Health 2020, 2, e504–e505. [Google Scholar] [CrossRef]
- Caulfield, T. Pseudoscience and COVID-19—We’ve had enough already. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Malik, M.; Raees, V.; Anwar, A. Role of Mass Media and Public Health Communications in the COVID-19 Pandemic. Cureus 2020, 12, e10453. [Google Scholar] [CrossRef]
- Dong, M.; Zheng, J. Letter to the editor: Headline stress disorder caused by Netnews during the outbreak of COVID-19. Health Expect. 2020, 23, 259–260. [Google Scholar] [CrossRef]
- Bendau, A.; Petzold, M.B.; Pyrkosch, L.; Mascarell Maricic, L.; Betzler, F.; Rogoll, J.; Große, J.; Ströhle, A.; Plag, J. Associations between COVID-19 related media consumption and symptoms of anxiety, depression and COVID-19 related fear in the general population in Germany. Eur. Arch. Psychiatry Clin. Neurosci. 2020. [Google Scholar] [CrossRef]
- Sasaki, N.; Kuroda, R.; Tsuno, K.; Kawakami, N. Exposure to media and fear and worry about COVID-19. Psychiatry Clin. Neurosci. 2020, 74, 501–502. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzymski, P.; Borkowski, L.; Drąg, M.; Flisiak, R.; Jemielity, J.; Krajewski, J.; Mastalerz-Migas, A.; Matyja, A.; Pyrć, K.; Simon, K.; et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines 2021, 9, 109. https://doi.org/10.3390/vaccines9020109
Rzymski P, Borkowski L, Drąg M, Flisiak R, Jemielity J, Krajewski J, Mastalerz-Migas A, Matyja A, Pyrć K, Simon K, et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines. 2021; 9(2):109. https://doi.org/10.3390/vaccines9020109
Chicago/Turabian StyleRzymski, Piotr, Leszek Borkowski, Marcin Drąg, Robert Flisiak, Jacek Jemielity, Jacek Krajewski, Agnieszka Mastalerz-Migas, Andrzej Matyja, Krzysztof Pyrć, Krzysztof Simon, and et al. 2021. "The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation" Vaccines 9, no. 2: 109. https://doi.org/10.3390/vaccines9020109
APA StyleRzymski, P., Borkowski, L., Drąg, M., Flisiak, R., Jemielity, J., Krajewski, J., Mastalerz-Migas, A., Matyja, A., Pyrć, K., Simon, K., Sutkowski, M., Wysocki, J., Zajkowska, J., & Fal, A. (2021). The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines, 9(2), 109. https://doi.org/10.3390/vaccines9020109