Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Silica from Rice Husk and Sugarcane Bagasse
2.3. Preparation of Membrane
2.4. Analysis of Membrane Morphology
2.5. Water Contact Angle Measurement
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Filtration Performance
3. Results and Discussion
3.1. Effects of Silica on Membrane Morphology
3.2. Membrane Hydrophilicity
3.3. Membrane Chemical Composition Analysis
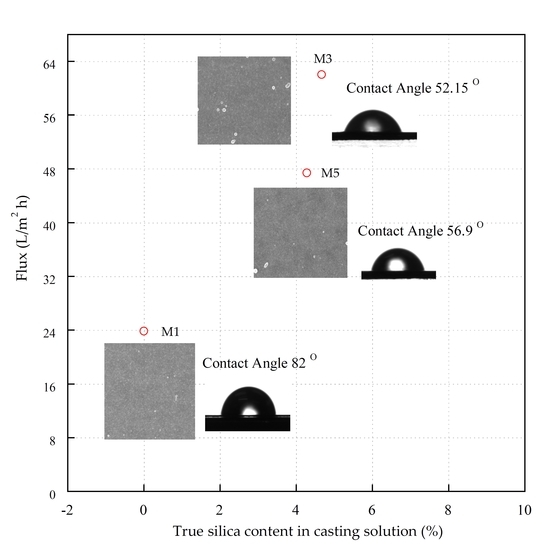
3.4. Filtration Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Chen, F. Hydrophilicity and antifouling property of membrane materials from cellulose acetate/polyethersulfone in DMAc. Int. J. Biol. Macromol. 2016, 91, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, S.; Wahab, M.Y.; Fang, L.; Jeon, S.; Rajabzadeh, S.; Takagi, R.; Mulyati, S.; Arahman, N.; Riza, M. Polydopamine-coated poly (vinylidene fl uoride) membranes with high ultraviolet resistance and antifouling properties for a photocatalytic membrane reactor. J. Appl. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Muchtar, S.; Yusuf, M.; Mulyati, S.; Arahman, N.; Riza, M. Superior fouling resistant PVDF membrane with enhanced fi ltration performance fabricated by combined blending and the self-polymerization approach of dopamine. J. Water Process Eng. 2019, 28, 293–299. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Rahmah, M.; Takagi, R.; Matsuyama, H. Removal pro file of sulfate ion from mix ion solution with diff erent type and configuration of anion exchange membrane in electrodialysis. J. Water Process Eng. 2017, 20, 173–179. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Aber, S. Improving the permeability and antifouling property of PES ultra fi ltration membranes using the drying method and incorporating the CuO-ZnO nanocomposite. J. Water Process Eng. 2019, 31, 100891. [Google Scholar] [CrossRef]
- Shen, L.; Bian, X.; Lu, X.; Shi, L.; Liu, Z.; Chen, L.; Hou, Z.; Fan, K. Preparation and characterization of ZnO/polyethersulfone (PES) hybrid membranes. Desalination 2019, 293, 21–29. [Google Scholar] [CrossRef]
- Garcia-ivars, J.; Alcaina-miranda, M.; Iborra-clar, M. Enhancement in hydrophilicity of different polymer phase-inversion ultrafiltration membranes by introducing PEG/Al2O3 nanoparticles. Sep. Purif. Technol. 2014, 128, 45–57. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, J.; Fang, B.; Nie, S.; Yi, N.; Su, B.; Li, H.; Zhao, C. Modification of polyethersulfone membrane by blending semi-interpenetrating network polymeric nanoparticles. J. Memb. Sci. 2011, 369, 258–266. [Google Scholar] [CrossRef]
- Li, F.; Meng, J.; Ye, J.; Yang, B.; Tian, Q.; Deng, C. Surface modi fi cation of PES ultra fi ltration membrane by polydopamine coating and poly (ethylene glycol) grafting: Morphology, stability, and anti-fouling. Desalination 2014, 344, 422–430. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Fahrina, A.; Muchtar, S. Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation. Molecules 2019, 24, 4099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, M.; Bilad, M.R.; Gebreyohannes, A.Y.; Marbelia, L.; Vankelecom, I.F.J. Membrane development for improved performance of a magnetically induced vibration system for anaerobic sludge fi ltration. Sep. Purif. Technol. 2018, 200, 120–129. [Google Scholar] [CrossRef]
- Zhang, D.; Karkooti, A.; Liu, L.; Sadrzadeh, M.; Thundat, T.; Liu, Y. Fabrication of antifouling and antibacterial polyethersulfone (PES)/cellulose nanocrystals (CNC) nanocomposite membranes. J. Memb. Sci. 2018, 549, 350–356. [Google Scholar] [CrossRef]
- Wahab, M.Y.; Muchtar, S.; Jeon, S.; Fang, L.; Rajabzadeh, S.; Takagi, R.; Arahman, N.; Mulyati, S.; Riza, M.; Matsuyama, H. Synergistic effects of organic and inorganic additives in preparation of composite poly (vinylidene fl uoride) antifouling ultra fi ltration membranes. J. Appl. Polym. Sci. 2019, 47737, 1–10. [Google Scholar]
- Gohari, R.J.; Halakoo, E.; Nazri, N.A.M.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Improving performance and antifouling capability of PES UF membranes via blending with highly hydrophilic hydrous manganese dioxide nanoparticles. Desalination 2014, 335, 87–95. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Lubis, M.R.; Razi, F.; Takagi, R.; Matsuyama, H. Modification of polyethersulfone hollow fiber membrane with different polymeric additives. Membr. Water Treat. 2016, 7, 355–365. [Google Scholar] [CrossRef]
- Shen, J.-N.; Ruan, H.-M.; Wu, L.-G.; Gao, C.-J. Preparation and characterization of PES-SiO2 organic-inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Idris, A.; Mat, N.; Noordin, M.Y. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 2007, 207, 324–339. [Google Scholar] [CrossRef]
- Kim, K.; Woo, S.H.; Lee, J.S.; Park, H.S.; Park, J. Improved Permeate Flux of PVDF Ultrafiltration Membrane Containing PVDF-g-PHEA Synthesized via ATRP. Appl. Sci. 2015, 5, 1992–2008. [Google Scholar] [CrossRef] [Green Version]
- Habibi, S.; Nematollahzadeh, A. Enhanced water flux through ultrafiltration polysulfone membrane via addition-removal of silica nano-particles: Synthesis and characterization. J. Appl. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Memb. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Bilad, M.R.; Kujawa, J.; Al-gharabli, S.; Arafat, H.A. On the effect of fumed silica particles on the structure, properties and application of PVDF membranes. Sep. Purif. Technol. 2017, 187, 365–373. [Google Scholar] [CrossRef]
- Kumar, S.; Guria, C.; Mandal, A. Synthesis, characterization and performance studies of polysulfone/bentonite nanoparticles mixed-matrix ultra-filtration membranes using oil field produced water. Sep. Purif. Technol. 2015, 150, 145–158. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultra fi ltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation and performance of polysulfone-sulfonated poly (ether ether ketone) blend ultrafiltration membranes. Part I. Appl. Surf. Sci. 2007, 253, 8705–8712. [Google Scholar] [CrossRef]
- Velu, S.; Muruganandam, L.; Arthanareeswaran, G. Effect of Solvents on Performance of Polyethersulfone Ultrafilteration Membranes for Separation of Metal Ions. Int. J. Chem. Anal. Sci. 2011, 2, 82–86. [Google Scholar]
- Lin, J.; Ye, W.; Zhong, K.; Shen, J.; Jullok, N.; Sotto, A.; Bruggen, B. Van Der Chemical Engineering and Processing: Process Intensi fi cation Enhancement of polyethersulfone (PES) membrane doped by monodisperse Stöber silica for water treatment. Chem. Eng. Process. 2016, 107, 194–205. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and Shape of Protein Molecules at the Nanometer Level Determined by Sedimentation, Gel Filtration, and Electron Microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; ISBN 0470854456. [Google Scholar]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]







| Composition | Silica Particle from Rice Husk (%) | Silica Particle from Sugarcane Bagasse Biomasses (%) |
|---|---|---|
| SiO2 | 93.4 | 85.6 |
| Al2O3 | 3.1 | 1.98 |
| CaO | 1.0 | 4.4 |
| K2O | 0.7 | 5.2 |
| MgO | 0.65 | 0.4 |
| Fe2O3 | 0.45 | 2.2 |
| NaO | 0.22 | - |
| Loss on ingnition | 0.48 | 0.22 |
| Membrane | PES (wt%) | Silica (wt%) | NMP (wt%) | ||
|---|---|---|---|---|---|
| Rice Husk | Bagasse | True Silica | |||
| M1 | 17.5 | 0 | 0 | 0 | 82.5 |
| M2 | 14.5 | 3 | 0 | 2.88 | 82.5 |
| M3 | 12.5 | 5 | 0 | 4.67 | 82.5 |
| M4 | 14.5 | 0 | 3 | 2.57 | 82.5 |
| M5 | 12.5 | 0 | 5 | 4.28 | 82.5 |
| Membrane | Lp (L/m2·h·bar) | Type of Membrane |
|---|---|---|
| M1 | 10.346 | NF/UF |
| M2 | 18.753 | NF/UF |
| M3 | 29.480 | NF/UF |
| M4 | 16.779 | NF/UF |
| M5 | 23.740 | NF/UF |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyati, S.; Muchtar, S.; Yusuf, M.; Arahman, N.; Sofyana, S.; Rosnelly, C.M.; Fathanah, U.; Takagi, R.; Matsuyama, H.; Shamsuddin, N.; et al. Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource. Membranes 2020, 10, 17. https://doi.org/10.3390/membranes10010017
Mulyati S, Muchtar S, Yusuf M, Arahman N, Sofyana S, Rosnelly CM, Fathanah U, Takagi R, Matsuyama H, Shamsuddin N, et al. Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource. Membranes. 2020; 10(1):17. https://doi.org/10.3390/membranes10010017
Chicago/Turabian StyleMulyati, Sri, Syawaliah Muchtar, Mukramah Yusuf, Nasrul Arahman, Sofyana Sofyana, Cut Meurah Rosnelly, Umi Fathanah, Ryosuke Takagi, Hideto Matsuyama, Norazanita Shamsuddin, and et al. 2020. "Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource" Membranes 10, no. 1: 17. https://doi.org/10.3390/membranes10010017
APA StyleMulyati, S., Muchtar, S., Yusuf, M., Arahman, N., Sofyana, S., Rosnelly, C. M., Fathanah, U., Takagi, R., Matsuyama, H., Shamsuddin, N., & Bilad, M. R. (2020). Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource. Membranes, 10(1), 17. https://doi.org/10.3390/membranes10010017