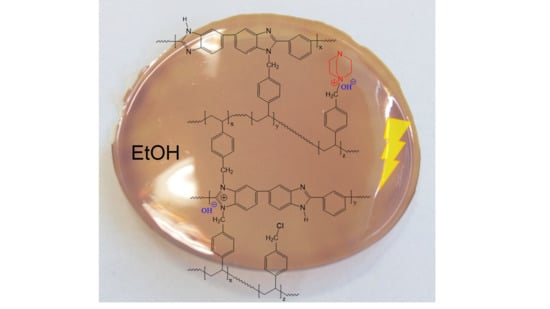
Application of Crosslinked Polybenzimidazole-Poly(Vinyl Benzyl Chloride) Anion Exchange Membranes in Direct Ethanol Fuel Cells
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Preparation Procedure of the Membranes
2.2. Structure Characterization and Morphology
2.3. Mechanical Properties, Chemical Stability, Volume Swelling, IEC and Conductivity
2.4. Single-Cell Performance Test
3. Results and Discussion
3.1. Structure Characterization and Morphology
3.1.1. XPS
3.1.2. SEM/EDX
3.1.3. Solid 13C-NMR
3.2. Mechanical Properties
3.3. Degradation in Oxidative Media
3.4. Performance in Alkaline Direct Ethanol Fuel Cell Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Galetti, M.; Alamgir, M.; Crist, E.; Mahmoud, M.I.; Laurance, W.F. World scientists’ warning to humanity: A second notice. Bioscience 2017, 67, 1026–1028. [Google Scholar] [CrossRef]
- Perera, A.T.D.; Nik, V.M.; Chen, D.; Scartezzini, J.L.; Hong, T. Quantifying the impacts of climate change and extreme climate events on energy systems. Nat. Energy 2020, 5, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Houchins, C.; Kleen, G.J.; Spendelow, J.S.; Kopasz, J.; Peterson, D.; Garland, N.L.; Ho, D.L.; Marcinkoski, J.; Martin, K.E.; Tyler, R.; et al. U.S. doe progress towards developing low-cost, high performance, durable polymer electrolyte membranes for fuel cell applications. Membranes 2012, 2, 855–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite membranes for high temperature PEM fuel cells and electrolysers: A critical review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Pletcher, D.; Li, X. Prospects for alkaline zero gap water electrolysers for hydrogen production. Int. J. Hydrog. Energy 2011, 36, 15089–15104. [Google Scholar] [CrossRef] [Green Version]
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion exchange membranes: Current status and moving forward. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Wu, Q.X.; Pan, Z.F.; An, L. Recent advances in alkali-doped polybenzimidazole membranes for fuel cell applications. Renew. Sustain. Energy Rev. 2018, 89, 168–183. [Google Scholar] [CrossRef]
- Kerres, J.A.; Krieg, H.M. Poly(Vinylbenzylchloride) based anion-exchange blend membranes (AEBMs): Influence of PEG additive on conductivity and stability. Membranes 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Chu, X.; Zhang, X.; Li, X.; Zheng, J.; Li, S.; Li, N.; Sherazi, T.A.; Zhang, S. The effect of polymer backbones and cation functional groups on properties of anion exchange membranes for fuel cells. J. Memb. Sci. 2020, 603, 118025. [Google Scholar] [CrossRef]
- Tuan, C.M.; Tinh, V.D.C.; Kim, D. Anion exchange membranes prepared from quaternized polyepichlorohydrin cross-linked with 1-(3-aminopropyl)imidazole grafted poly(arylene ether ketone) for enhancement of toughness and conductivity. Membranes 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Zakaria, Z. Enhanced alkaline stability and performance of alkali-doped quaternized poly(vinyl alcohol) membranes for passive direct ethanol fuel cell. Int. J. Energy Res. 2019, 43, 5252–5265. [Google Scholar] [CrossRef]
- Hao, J.; Jiang, Y.; Gao, X.; Lu, W.; Xiao, Y.; Shao, Z.; Yi, B. Functionalization of polybenzimidazole-crosslinked poly ( vinylbenzyl chloride ) with two cyclic quaternary ammonium cations for anion exchange membranes. J. Memb. Sci. 2018, 548, 1–10. [Google Scholar] [CrossRef]
- Yu, S.; Ma, X.; Liu, H.; Hao, J. Highly stable double crosslinked membrane based on poly ( vinylbenzyl chloride ) for anion exchange membrane fuel cell. Polym. Bull. 2018, 75, 5163–5177. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, G.; Li, J.; Hao, J.; Wei, F.; Li, W.; Zhang, J.; Shao, Z.-G.; Yi, B. Polybenzimidazole-crosslinked poly(vinylbenzyl chloride) with quaternary 1,4-diazabicyclo (2.2.2) octane groups as high-performance anion exchange membrane for fuel cells. J. Power Sources 2015, 296, 204–214. [Google Scholar] [CrossRef]
- Hao, J.; Gao, X.; Jiang, Y.; Xie, F.; Shao, Z.; Yi, B. Fabrication of N1-butyl substituted 4,5-dimethylimidazole based crosslinked anion exchange membranes for fuel cells. RSC Adv. 2017, 7, 52812–52821. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; He, H.; Hsu, A.; Chen, R. PdRu/C catalysts for ethanol oxidation in anion-exchange membrane direct ethanol fuel cells. J. Power Sources 2013, 241, 696–702. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Li, M.J. Highly Dispersed Palladium Nanoparticles on Carbon-Decorated Porous Nickel Electrode: An Effective Strategy to Boost Direct Ethanol Fuel Cell up to 202 mW cm-2. ACS Sustain. Chem. Eng. 2019, 7, 11186–11193. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Jiang, Q.; Jin, W.; Jiang, L.; Sun, G. Durability study of KOH doped polybenzimidazole membrane for air-breathing alkaline direct ethanol fuel cell. J. Power Sources 2011, 196, 3244–3248. [Google Scholar] [CrossRef]
- Hou, H.; Sun, G.; He, R.; Wu, Z.; Sun, B. Alkali doped polybenzimidazole membrane for high performance alkaline direct ethanol fuel cell. J. Power Sources 2008, 182, 95–99. [Google Scholar] [CrossRef]
- Modestov, A.D.; Tarasevich, M.R.; Leykin, A.Y.; Filimonov, V.Y. MEA for alkaline direct ethanol fuel cell with alkali doped PBI membrane and non-platinum electrodes. J. Power Sources 2009, 188, 502–506. [Google Scholar] [CrossRef]
- Coppola, R.E.; Herranz, D.; Escudero-Cid, R.; Ming, N.; D’Accorso, N.B.; Ocón, P.; Abuin, G.C. Polybenzimidazole-crosslinked-poly(vinyl benzyl chloride) as anion exchange membrane for alkaline electrolyzers. Renew. Energy 2020, 157, 71–82. [Google Scholar] [CrossRef]
- Herranz, D.; Escudero-Cid, R.; Montiel, M.; Palacio, C.; Fatás, E.; Ocón, P. Poly (vinyl alcohol) and poly (benzimidazole) blend membranes for high performance alkaline direct ethanol fuel cells. Renew. Energy 2018, 127, 883–895. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xing, T.; Sun, B.; Feng, Z.; Li, P.; Yang, Z.; Li, S.; Chen, S. Effects of salt concentration on the structure and properties of composite fiber of carboxymethyl cellulose/N-2-hydroxylpropyl trimethyl ammonium chloride chitosan prepared by polyelectoyte complexation-freeze drying. Int. J. Biol. Macromol. 2020, 151, 1030–1039. [Google Scholar] [CrossRef]
- Escaro, J.; Mavel, G.; Guerchais, J.E.; Kergoat, R. X-Ray Photoelectron Spectroscopy Study of Some Metal(II) Halide and Pseudohalide Complexes. Inorg. Chem. 1974, 13, 695–701. [Google Scholar] [CrossRef]
- Law, R.V.; Sherrington, D.C.; Snape, C.E.; Ando, I.; Kurosu, H. Solid-State 13 C MAS NMR Studies of Hyper-Cross-Linked Polystyrene Resins. Macromolecules 1996, 29, 6284–6293. [Google Scholar] [CrossRef]
- Fontanals, N.; Cortés, J.; Galià, M.; Marcé, R.M.; Cormack, P.A.G.; Borrull, F.; Sherrington, D.C. Synthesis of davankov-type hypercrosslinked resins using different isomer compositions of vinylbenzyl chloride monomer, and application in the solid-phase extraction of polar compounds. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1718–1728. [Google Scholar] [CrossRef]
- Lohar, T.; Kumbhar, A.; Patil, A.; Kamat, S.; Salunkhe, R. Synthesis and characterization of new quaternary ammonium surfactant [C 18 -Dabco][Br] and its catalytic application in the synthesis of spirocarbocycles under ultrasonic condition. Res. Chem. Intermed. 2019, 45, 1639–1651. [Google Scholar] [CrossRef]
- Narducci, R.; Chailan, J.F.; Fahs, A.; Pasquini, L.; Di Vona, M.L.; Knauth, P. Mechanical properties of anion exchange membranes by combination of tensile stress-strain tests and dynamic mechanical analysis. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1180–1187. [Google Scholar] [CrossRef]
- Taylor, P.; Kim, Y.S.; Lee, K. Fuel Cell Membrane Characterizations. Polymer Rev. 2015, 55, 37–41. [Google Scholar] [CrossRef]
- Hao, J.; Jiang, Y.; Gao, X.; Xie, F.; Shao, Z.; Yi, B. Degradation reduction of polybenzimidazole membrane blended with CeO2 as a regenerative free radical scavenger. J. Memb. Sci. 2017, 522, 23–30. [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Akhmadiyarov, A.A.; Vandyukov, A.E.; Khamatgalimov, A.R. Experimental vibrational spectra DABCO. J. Mol. Struct. 2012, 1028, 134–140. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]












| Conductivity 90 °C (mS·cm−1) | IEC (mmol·g−1) | IEC (%) | Water Uptake (%) | Vol. Swelling (%) | |
|---|---|---|---|---|---|
| PBI-c-PVBC/OH 1:2 | 30 | 1.74 | (−)10 | (+)20 | (+)18 |
| PBI-c-PVBC/OH 1:3 | 45 | 1.97 | (−)16 | (+)11 | (+)30 |
| Membrane | Thickness (µm) | Average Power Density (mW cm−2) | Temperature (°C) | O2 Backpressure (bar) |
|---|---|---|---|---|
| PBI-c-PVBC/OH 1:1 | 36 | 32 | 90 | 3 |
| PBI-c-PVBC/OH 1:2 | 32 | 70 | 90 | 2 |
| PBI-c-PVBC/OH 1:3 | 38 | 49 | 90 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz, D.; Coppola, R.E.; Escudero-Cid, R.; Ochoa-Romero, K.; D’Accorso, N.B.; Pérez-Flores, J.C.; Canales-Vázquez, J.; Palacio, C.; Abuin, G.C.; Ocón, P. Application of Crosslinked Polybenzimidazole-Poly(Vinyl Benzyl Chloride) Anion Exchange Membranes in Direct Ethanol Fuel Cells. Membranes 2020, 10, 349. https://doi.org/10.3390/membranes10110349
Herranz D, Coppola RE, Escudero-Cid R, Ochoa-Romero K, D’Accorso NB, Pérez-Flores JC, Canales-Vázquez J, Palacio C, Abuin GC, Ocón P. Application of Crosslinked Polybenzimidazole-Poly(Vinyl Benzyl Chloride) Anion Exchange Membranes in Direct Ethanol Fuel Cells. Membranes. 2020; 10(11):349. https://doi.org/10.3390/membranes10110349
Chicago/Turabian StyleHerranz, Daniel, Roxana E. Coppola, Ricardo Escudero-Cid, Kerly Ochoa-Romero, Norma B. D’Accorso, Juan Carlos Pérez-Flores, Jesús Canales-Vázquez, Carlos Palacio, Graciela C. Abuin, and Pilar Ocón. 2020. "Application of Crosslinked Polybenzimidazole-Poly(Vinyl Benzyl Chloride) Anion Exchange Membranes in Direct Ethanol Fuel Cells" Membranes 10, no. 11: 349. https://doi.org/10.3390/membranes10110349
APA StyleHerranz, D., Coppola, R. E., Escudero-Cid, R., Ochoa-Romero, K., D’Accorso, N. B., Pérez-Flores, J. C., Canales-Vázquez, J., Palacio, C., Abuin, G. C., & Ocón, P. (2020). Application of Crosslinked Polybenzimidazole-Poly(Vinyl Benzyl Chloride) Anion Exchange Membranes in Direct Ethanol Fuel Cells. Membranes, 10(11), 349. https://doi.org/10.3390/membranes10110349