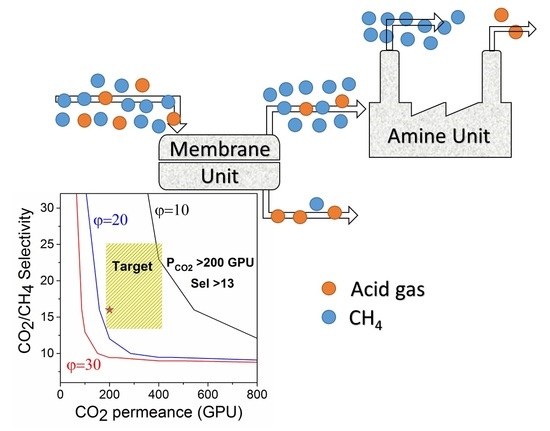
Superglassy Polymers to Treat Natural Gas by Hybrid Membrane/Amine Processes: Can Fillers Help?
Abstract
:1. Introduction
- acid gas permeance
- CO2/CH4 and H2S/CH4 selectivity
- pressure ratio between feed and permeate side
- feed gas flow rate
- acid gas content in the feed
- acid gas removal fraction in the membrane process
- hydrocarbon content (C2+) in the feed gas
- membrane process design, and
- membrane module design
2. Case Study
2.1. Gas Condition
2.2. Membrane Performance
2.3. Amine Solvent Types and Selection
- the capability of removing carbonyl sulfide (COS) and mercaptans from gas beside H2S and CO2:
- the DGA concentration in solution is usually 50 to 60% higher than other amines;
- a higher amine concentration means higher acid gas uptake, lower circulation rate, and lower electric power and heating duty requirements [1]; and
- the corrosion in the process using DGA solvent is slightly less than when using the MEA [7].
2.4. Total Capital Investment (TCI)
2.4.1. Amine Process Capital Cost
2.4.2. Membrane Unit Capital Cost
2.4.3. Fixed Capital Investment (FCI)
2.4.4. Working Capital Investment (WCI)
2.5. Operating Expenses
2.5.1. Membrane Operating Cost
2.5.2. Amine Operating Cost
2.5.3. Other General Operating Expenses
2.6. Cash Flow and Revenue
3. Results and Discussion
3.1. Membrane Area and Skid Cost
3.2. Total Capital Investment
3.3. Total Annual Operating Cost
3.4. Revenue and Hydrocarbon Losses
3.5. Net Present Value (NPV)
3.6. Effect of Feed Flowrate and Acid Gas Content
- (i)
- CO2 permeance = 200 GPU & CO2/CH4 selectivity = 16 (target performance)
- (ii)
- CO2 permeance = 400 GPU & CO2/CH4 selectivity = 16 (doubled CO2 permeance)
- (iii)
- CO2 permeance = 200 GPU & CO2/CH4 selectivity = 32 (doubled CO2/CH4 selectivity)
3.7. Sensitivity Factors
3.7.1. Ethane Content in the Feed
3.7.2. Membrane Costs (Skid, Material, Replacement Rate)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bahadori, A. Natural Gas Processing: Technology and Engineering Design; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Hugman, R.H.; Vidas, E.H.; Springer, P.S. Chemical Composition of Discovered and Undiscovered Natural Gas in the Lower-48 United States. Project Summary. Final Report, 1 November 1988–31 March 1990; Energy and Environmental Analysis, Inc.: Arlington, VA, USA, 1990. [Google Scholar]
- Mokhatab, S. Handbook of Natural Gas Transmission and Processing: Principles and Practices, 4th ed.; Gulf Professional Publishing: Oxford, UK, 2019. [Google Scholar]
- EIA. International Energy Outlook 2019 with Projections to 2050; EIA: Washington, DC, USA, 2019. [Google Scholar]
- IEA. Resources to Reserves 2013; IEA: Paris, France, 2013. [Google Scholar]
- Spillman, R. Economics of Gas Separation Membrane Processes. In Membrane Separations Technology—Principles and Applications; Noble, R.D., Stern, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 589–667. [Google Scholar]
- Stewart, M. Gas Sweetening and Processing Field Manual; Arnold, K., Ed.; Gulf Professional Pubulishing: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Dortmundt, D.; Doshi, K. Recent Developments in CO2 Removal Membrane Technology; UOP LLC: Des Plaines, IL, USA, 1999. [Google Scholar]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Newark, CA, USA, 2012. [Google Scholar]
- White, L.S. Evolution of Natural Gas Treatment with Membrane Systems. In Membrane Gas Separation; Yampolskii, Y., Freeman, B.D., Eds.; Wiley: Hoboken, NJ, USA, 2010; pp. 313–332. [Google Scholar]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T.; Takada, K. Poly [1-(trimethylsilyl)-1-propyne]: A new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474. [Google Scholar] [CrossRef]
- Srinivasan, R.; Auvil, S.R.; Burban, P.M. Elucidating the mechanism(s) of gas transport in poly[1-(trimethylsilyl)-1-propyne] (PTMSP) membranes. J. Membr. Sci. 1994, 86, 67–86. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Staiger, C.L.; Pas, S.J.; Hill, A.J.; Cornelius, C.J. Gas Separation, Free Volume Distribution, and Physical Aging of a Highly Microporous Spirobisindane Polymer. Chem. Mater. 2008, 20, 2606–2608. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Jin, J.; Freeman, B.D.; Paul, D.R. Physical aging, CO2 sorption and plasticization in thin films of polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2017, 537, 362–371. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Jansen, J.C.; et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Khdhayyer, M.; Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jiang, D.; Burrows, A.D.; Esposito, E.; Bernardo, P.; Monteleone, M.; Fuoco, A.; et al. Mixed matrix membranes based on MIL-101 metal–organic frameworks in polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2019, 212, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. Permselectivity improvement in membranes for CO2/N2 separation. Sep. Purif. Technol. 2016, 157, 102–111. [Google Scholar]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Song, J.; Du, N.; Robertson, G.P.; Guiver, M.D. Gas transport behavior of mixed-matrix membranes composed of silica nanoparticles in a polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2010, 346, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Alberto, M.; Bhavsar, R.; Luque-Alled, J.M.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Impeded physical aging in PIM-1 membranes containing graphene-like fillers. J. Membr. Sci. 2018, 563, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The influence of few-layer graphene on the gas permeability of the high-free-volume polymer PIM-1. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150031. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.; Sprouster, D.J.; et al. Ending Aging in Super Glassy Polymer Membranes. Angew. Chem. Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.Y.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-Separation Membranes Loaded with Porous Aromatic Frameworks that Improve with Age. Angew. Chem. 2015, 127, 2707–2711. [Google Scholar] [CrossRef]
- Mitra, T.; Bhavsar, R.S.; Adams, D.J.; Budd, P.M.; Cooper, A.I. PIM-1 mixed matrix membranes for gas separations using cost-effective hypercrosslinked nanoparticle fillers. Chem. Commun. 2016, 52, 5581–5584. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.H.; Mulet, X.; Konstas, K.; Doherty, C.M.; Sani, M.-A.; Separovic, F.; Hill, M.R.; Wood, C.D. Hypercrosslinked Additives for Ageless Gas-Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 1998–2001. [Google Scholar] [CrossRef]
- Tamaddondar, M.; Foster, A.B.; Luque-Alled, J.M.; Msayib, K.J.; Carta, M.; Sorribas, S.; Gorgojo, P.; McKeown, N.B.; Budd, P.M. Intrinsically Microporous Polymer Nanosheets for High-Performance Gas Separation Membranes. Macromol. Rapid Commun. 2020, 41, 1900572. [Google Scholar] [CrossRef]
- Tamaddondar, M.; Foster, A.B.; Carta, M.; Gorgojo, P.; McKeown, N.B.; Budd, P.M. Mitigation of Physical Aging with Mixed Matrix Membranes Based on Cross-Linked PIM-1 Fillers and PIM-1. Acs Appl. Mater. Interfaces 2020, 12, 46756–46766. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nothling, M.D.; Webley, P.A.; Jin, J.; Fu, Q.; Qiao, G.G. High-throughput CO2 capture using PIM-1@MOF based thin film composite membranes. Chem. Eng. J. 2020, 396, 125328. [Google Scholar] [CrossRef]
- Bakhtin, D.S.; Kulikov, L.A.; Legkov, S.A.; Khotimskiy, V.S.; Levin, I.S.; Borisov, I.L.; Maksimov, A.L.; Volkov, V.V.; Karakhanov, E.A.; Volkov, A.V. Aging of thin-film composite membranes based on PTMSP loaded with porous aromatic frameworks. J. Membr. Sci. 2018, 554, 211–220. [Google Scholar] [CrossRef]
- Koschine, T.; Rätzke, K.; Faupel, F.; Khan, M.M.; Emmler, T.; Filiz, V.; Abetz, V.; Ravelli, L.; Egger, W. Correlation of gas permeation and free volume in new and used high free volume thin film composite membranes. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 213–217. [Google Scholar] [CrossRef]
- Jue, M.L.; Breedveld, V.; Lively, R.P. Defect-free PIM-1 hollow fiber membranes. J. Membr. Sci. 2017, 530, 33–41. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Mitra, T.; Adams, D.J.; Cooper, A.I.; Budd, P.M. Ultrahigh-permeance PIM-1 based thin film nanocomposite membranes on PAN supports for CO2 separation. J. Membr. Sci. 2018, 564, 878–886. [Google Scholar] [CrossRef]
- Liang, C.Z.; Liu, J.T.; Lai, J.Y.; Chung, T.S. High-performance multiple-layer PIM composite hollow fiber membranes for gas separation. J. Membr. Sci. 2018, 563, 93–106. [Google Scholar] [CrossRef]
- Putintseva, M.N.; Borisov, I.L.; Yushkin, A.A.; Kirk, R.; Budd, P.M.; Volkov, A.V. Effect of Casting Solution Composition on Properties of PIM-1/PAN Thin Film Composite Membranes. Key Eng. Mater. 2019, 816, 167–173. [Google Scholar] [CrossRef]
- Borisov, I.; Bakhtin, D.; Luque-Alled, J.M.; Rybakova, A.; Makarova, V.; Foster, A.B.; Harrison, W.J.; Volkov, V.; Polevaya, V.; Gorgojo, P.; et al. Synergistic enhancement of gas selectivity in thin film composite membranes of PIM-1. J. Mater. Chem. A 2019, 7, 6417–6430. [Google Scholar] [CrossRef] [Green Version]
- Merkel, T.C.; Toy, L.G. Comparison of Hydrogen Sulfide Transport Properties in Fluorinated and Nonfluorinated Polymers. Macromolecules 2006, 39, 7591–7600. [Google Scholar] [CrossRef]
- Alghannam, A.A.; Yahaya, G.O.; Hayek, A.; Mokhtari, I.; Saleem, Q.; Sewdan, D.A.; Bahamdan, A.A. High pressure pure- and mixed sour gas transport properties of Cardo-type block co-polyimide membranes. J. Membr. Sci. 2018, 553, 32–42. [Google Scholar] [CrossRef]
- Liu, G.; Chernikova, V.; Liu, Y.; Zhang, K.; Belmabkhout, Y.; Shekhah, O.; Zhang, C.; Yi, S.; Eddaoudi, M.; Koros, W.J. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat. Mater. 2018, 17, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hayek, A.; Yahaya, G.O.; Alsamah, A.; Alghannam, A.A.; Jutaily, S.A.; Mokhtari, I. Pure-and sour mixed-gas transport properties of 4,4′-methylenebis(2,6-diethylaniline)-based copolyimide membranes. Polymer 2019, 166, 184–195. [Google Scholar] [CrossRef]
- Yi, S.; Ghanem, B.; Liu, Y.; Pinnau, I.; Koros, W.J. Ultraselective glassy polymer membranes with unprecedented performance for energy-efficient sour gas separation. Sci. Adv. 2019, 5, eaaw5459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhide, B.D.; Voskericyan, A.; Stern, S.A. Hybrid processes for the removal of acid gases from natural gas. J. Membr. Sci. 1998, 140, 27–49. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Heydari, I.; Zhang, Z. Hybrid systems: Combining membrane and absorption technologies leads to more efficient acid gases (CO2 and H2S) removal from natural gas. J. Co2 Util. 2017, 18, 362–369. [Google Scholar] [CrossRef]
- Hamad, F.; Qahtani, M.; Ameen, A.; Vaidya, M.; Duval, S.; Bahamdan, A.; Otaibi, F. Treatment of highly sour natural gas stream by hybrid membrane-amine process: Techno-economic study. Sep. Purif. Technol. 2020, 237, 116–348. [Google Scholar] [CrossRef]
- Thomas, S.; Pinnau, I.; Du, N.; Guiver, M.D. Hydrocarbon/hydrogen mixed-gas permeation properties of PIM-1, an amorphous microporous spirobisindane polymer. J. Membr. Sci. 2009, 338, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley & Sons: Hoboken, NJ, USA, 1974. [Google Scholar]
- Freeman, B.D. Membrane Gas Separation; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Bae, H.K.; Kim, S.Y.; Lee, B. Simulation of CO2 removal in a split-flow gas sweetening process. Korean J. Chem. Eng. 2011, 28, 643–648. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R.K. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Coker, A.K. Ludwig’s Applied Process Design for Chemical and Petrochemical Plants; Gulf Professional Publishing: United States, 2007. [Google Scholar]
- Joint Working Party of the Institution of Chemical Engineers, and The Association of Cost Engineers. A New Guide to Capital Cost Estimating; Institution of Chemical Engineers: London, UK, 1977. [Google Scholar]
- Peter, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers; McGraw-Hill: Boston, MA, USA, 2004. [Google Scholar]
- Peter, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineers, 4th ed.; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Scholes, C.A. Cost Competitive Membrane Processes for Carbon Capture. In Membrane Engineering for the Treatment of Gases-Vo-1; Drioli, E., Barbieri, G., Brunetti, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 216–241. [Google Scholar]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Coker, A.K. Ludwig’s Applied Process Design for Chemical and Petrochemical Plants: Volume 2: Distillation, Packed Towers, Petroleum Fractionation, Gas Processing and Dehydration; Elsevier Science: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Gas Processors Suppliers Association. Engineering Data Book, 12th ed.; Publisher: Tulsa, OK, USA, 2004; Volume 1–2. [Google Scholar]
- El-Halwagi, M.M. Sustainable Design through Process Integration: Fundamentals and Applications to Industrial Pollution Prevention, Resource Conservation, and Profitability Enhancement, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Lee, A.L.; Feldkirchner, H.L.; Stern, S.A.; Houde, A.Y.; Gamez, J.P.; Meyer, H.S. Field tests of membrane modules for the separation of carbon dioxide from low-quality natural gas. Gas Sep. Purif. 1995, 9, 35–43. [Google Scholar] [CrossRef]
- Schell, W.J.; Wensley, C.G.; Chen, M.S.K.; Venugopal, K.G.; Miller, B.D.; Stuart, J.A. Recent advances in cellulosic membranes for gas separation and pervaporation. Gas Sep. Purif. 1989, 3, 162–169. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas-separation technology. Membr. Technol. 2001, 2001, 5–10. [Google Scholar] [CrossRef]











| Selective Layer | Gutter Layer | Support | Membrane Configuration and Thickness | Testing Pressure | CO2 Permeance [GPU] | N2 Permeance [GPU] | Estimated CH4 Permeance * [GPU] | CO2/CH4 Selectivity | Aging Time | Reference | Year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTMSP/PAF11 | - | PAN | Flat (kiss coating) 6.8 μm | 2 bar | 1900 | 280 | 350 | 5.4 | 450 days | [33] | 2018 |
| PTMSP | - | PAN | Flat (kiss coating) 2.8 μm | 2 bar | 310 | 19 | 24 | 13.1 | 600 days | ||
| PTMSP/PAF11 | - | PAN | Flat (kiss coating) 1.7 μm | 2 bar | 500 | 31 | 39 | 12.9 | 650 days | ||
| PIM-1 | - | PAN | Flat (dip coating) 0.7 μm | 2 bar | 3880 ** | 110 ** | 138 | 28.2 | 300 days | [34] | 2015 |
| f-MWCNTs/PIM-1 | - | PAN | Flat (dip coating) 0.7 μm | 2 bar | 8200 ** | 220 ** | 275 | 29.8 | 300 days | ||
| PIM-1 | - | HF (spinning) 2.8 μm | 100 psi | 360 | 13 | 16 | 22.2 | 2 months | [35] | 2017 | |
| PIM-1 | - | PAN | Flat (coating) | 2 atm | 388 | 16 | 20 | 19.4 | 90 days | [36] | 2018 |
| PIM-1/C-HCP (40 wt%) | - | PAN | Flat (coating) | 2 atm | 9379 | 834 | 1043 | 9.0 | 90 days | ||
| PIM-1 | PDMS | PAN | HF (coating) | 2 bar | 402.6 | 18.9 | 24 | 17.0 | Fresh | [37] | 2018 |
| PIM-1 (4 wt% in CHCl3 and amylene) | - | PAN | Flat (kiss coating) 6 μm | 0.5–5 bar | 144 | 3.7 | 5 | 31.1 | Fresh | [38] | 2019 |
| PIM-1 (2 wt% in CHCl3 and EtOH) | - | PAN | Flat (kiss coating) 2.7 μm | 0.5–5 bar | 415.9 | 14.4 | 18 | 23.1 | Fresh | ||
| PIM-1 | Cross-linked PTMSP | MFFK-1 | Flat (kiss coating) 0.29–0.42 μm | 3 bar | 208–297 | 6 to 8 | 8 to 10 | 34 to 56 | 95 days | [39] | 2019 |
| PIM-1 | PDMS/MOF | PAN | Flat (spinning) | 2 bar | 490 | 16 | 20 | 24.5 | 8 weeks | [32] | 2020 |
| PIM-1/MOF-74-Ni | PDMS/MOF | PAN | Flat (spinning) | 2 bar | 1200 | 40 | 50 | 24.0 | 8 weeks | ||
| PIM-1/NH2-UiO-66 | PDMS/MOF | PAN | Flat (spinning) | 2 bar | 900 | 35 | 44 | 20.6 | 8 weeks |
| Feed Flow [MMSCFD] | 100 | |
| Temperature [°F] | 120 | |
| Feed pressure [psia] | 900/600/300 | |
| Permeate pressure [psia] | 30 | |
| Composition | CH4 | 56.0% |
| CO2 | 8.0% | |
| N2 | 10.0% | |
| H2S | 20.0% | |
| H2O | 0.1% | |
| C2H6 | 5.0% | |
| C3H8 | 0.9% |
| Equipment | Cost Exponent Factor |
|---|---|
| Absorber | 1 |
| Regenerator | 1 |
| Heat exchangers | 0.8 |
| Pumps/Air cooler fans | 0.8 |
| Surge tanks | 0.6 |
| Purchased Equipment | 1 | |
| Delivered cost factor | 0.1 | |
| Component | Factor | |
| Direct cost factor | Equipment erection | 0.40 |
| Piping | 0.65 | |
| Instrumentation and control | 0.30 | |
| Electrical | 0.25 | |
| Process buildings and structures | 0.45 | |
| Service facility | 0.75 | |
| Site preparation | 0.15 | |
| Indirect cost factor | Engineering/Supervision | 0.33 |
| Construction expenses | 0.40 | |
| Legal expenses | 0.10 | |
| Contractors fee | 0.10 | |
| Contingency | 0.40 | |
| Total factor = Direct cost factor + Indirect cost factor + Delivered cost factor | 5.38 | |
| FCI = Total factor × Equipment cost WCI = OPEX (first year) + Amine loading + start-up expenses TCI = FCI + WCI | ||
| [× Amine Circulation Rate (GPM)] | |
|---|---|
| Reboiler [MMBtu/h] | 0.072 |
| Rich/lean amine heat exchanger [MMBtu/h] | 0.045 |
| Amine cooler [MMBtu/h] | 0.015 |
| Reflux condenser [MMBtu/h] | 0.03 |
| [× Amine Circulation Rate (GPM)] | |
|---|---|
| Main amine pump [hp] | 6.5 × 10‒4 × feed pressure (psig) |
| Amine booster pumps [hp] | 0.06 |
| Reflux pumps [hp] | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, A.W.; Budd, P.M.; Gorgojo, P. Superglassy Polymers to Treat Natural Gas by Hybrid Membrane/Amine Processes: Can Fillers Help? Membranes 2020, 10, 413. https://doi.org/10.3390/membranes10120413
Ameen AW, Budd PM, Gorgojo P. Superglassy Polymers to Treat Natural Gas by Hybrid Membrane/Amine Processes: Can Fillers Help? Membranes. 2020; 10(12):413. https://doi.org/10.3390/membranes10120413
Chicago/Turabian StyleAmeen, Ahmed W., Peter M. Budd, and Patricia Gorgojo. 2020. "Superglassy Polymers to Treat Natural Gas by Hybrid Membrane/Amine Processes: Can Fillers Help?" Membranes 10, no. 12: 413. https://doi.org/10.3390/membranes10120413
APA StyleAmeen, A. W., Budd, P. M., & Gorgojo, P. (2020). Superglassy Polymers to Treat Natural Gas by Hybrid Membrane/Amine Processes: Can Fillers Help? Membranes, 10(12), 413. https://doi.org/10.3390/membranes10120413