Effect of Loading and Functionalization of Carbon Nanotube on the Performance of Blended Polysulfone/Polyethersulfone Membrane during Treatment of Wastewater Containing Phenol and Benzene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blended PSF/PES Membrane
2.3. Purification and Functionalization of CNTs
2.4. Preparation of CNT/Blended Membranes
2.5. Equilibrium Water Content (EWC) and Porosity
2.6. Membrane Characterization
2.7. Membrane Performance
3. Results and Discussion
3.1. Effect of Incorporation of CNT on Membrane EWC and Porosity
3.2. Effect of CNTs on Thermal Stability
3.3. Effect of CNTs on Membrane Tensile Strength
3.4. Effect of CNTs on Surface Nature of the Membrane
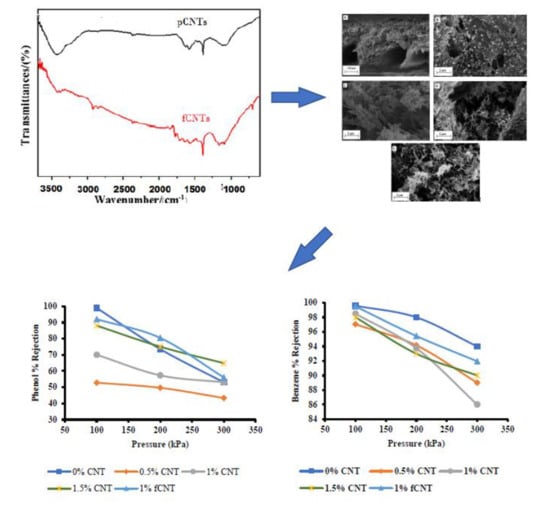
3.5. Effect of CNT Loading on Morphology
3.6. Effect of CNT on Pure Water Flux (PWF) and Removal of Phenol and Benzene from Wastewater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mushtaq, A.; Mukhtar, H.B.; Shariff, A.M.; Mannan, H.A. A Review: Fabrication of Enhanced Polymeric Blend Membrane with Amines for Removal of CO2 from Natural Gas. IJETAE 2013, 3, 21–34. [Google Scholar]
- Mendez, M.L.; Romero, I.A.; Rajal, V.B.; Castro, E.F.; Calvo, J.I.; Palacio, L.; Hernandez, A. Properties of Polyethersulfone Ultrafiltration Membranes Modified with Polyethylene Glycols. Polym Eng Sci. 2013. [Google Scholar] [CrossRef]
- Rashid, M.H.; Ralph, S.F. Carbon Nanotube Membranes: Synthesis, Properties, and Future Filtration Applications. Nomater 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, K.; Zhang, H.; Gu, Y. Graphene and carbon nanotube hybrid structure: A review. Procedia IUTAM 2017, 21, 94–101. [Google Scholar] [CrossRef]
- Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of Carbon Nanotube/Polysulfone (CNT/PSF) composite membranes during oil-water mixture separation: Effect of CNT dispersion method. Membranes 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.H.; Bai, J.; Cheng, H.M. The present status and key problems of carbon nanotube-based polymer composites. Express Polym. Lett. 2007, 1, 253–273. [Google Scholar] [CrossRef]
- Kochkodan, V.; Johnson, D.J.; Hilal, N. Polymeric membranes: Surface modification for minimizing (bio)colloidal fouling. Adv. Colloid Interface Sci. 2014, 206, 116–140. [Google Scholar] [CrossRef]
- Rameetse, M.S.; Aberefa, O.; Daramola, M.O. Synthesis and characterization of PSF/PES composite membranes for use in oily wastewater treatment. J. Phys. Conf. Ser. 2019. [Google Scholar] [CrossRef]
- Luo, Z.; Oki, A.; Carson, L.; Adams, L.; Neelgund, G.; Soboyejo, N.; Regisford, G.; Stewart, M.; Hibbert, K.; Beharie, G.; et al. Thermal stability of functionalized carbon nanotubes studied by in-situ transmission electron microscopy. Chem. Phys. Lett. 2011, 6, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wang, M.; Zhang, W.; Liu, T. Preparation and characterization of poly (vinylidene fluoride) nanocomposites containing multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2009, 113, 644–650. [Google Scholar] [CrossRef]
- Xu, L.; He, J.; Yu, Y.; Chen, J.P. Effect of CNT content on physicochemical properties and performance of CNT composite polysulfone membranes. Chem. Eng. Res. Des. 2017, 121, 92–98. [Google Scholar] [CrossRef]
- Esbati, A.H.; Irani, S. Effect of functionalized process and CNTs aggregation on fracture mechanism and mechanical properties of polymer nanocomposite. Mech. Mater. 2018, 118, 106–119. [Google Scholar] [CrossRef]
- Shima, H. Buckling of carbon nanotubes: A state of the art review. Materials 2011, 5, 47–84. [Google Scholar] [CrossRef] [Green Version]
- Phasha, J. Synthesis of Carbon Nanotubes–polyphenylene Sulfone Composite Membranes for Wastewater Treatment from Petroleum Sources. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2017. [Google Scholar]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jegal, J.; Kim, W.N. Fabrication and characterization of multi-walled carbon nanotubes/polymer membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Mehrabadi, M.M.; Aghaei, A.; Yaghmaee, M.S.; Karimnezhad, M.; Kamari, M. The effect of functional carbon nanotubes on the permeability of ultrafiltration nanocomposite PVDF membranes. J. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity. J. Appl. Polym. Sci. 2015, 132, 42042–42053. [Google Scholar] [CrossRef]
- Powell, L.C.; Hilal, N.; Wright, C.J. Atomic force microscopy study of the biofouling and mechanical properties of virgin and industrially fouled reverse osmosis membranes. Desalination 2017, 404, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Najjar, A.; Sabri, S.; Al-Gaashani, R.; Atieh, M.A.; Kochkodan, V. Antibiofouling Performance by Polyethersulfone Membranes Cast with Oxidized Multiwalled Carbon Nanotubes and Arabic Gum. Membranes 2019, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Deng, B.J. Polymer matrix nanocomposite membranes for water treatment. Membr. Sci. 2014, 11, 256–275. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Mai, W. Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J. Mater. Chem. 2013, 1, 3101–3111. [Google Scholar] [CrossRef]
- Feng, Y.; Shamsaei, E.; Davies, C.H.J.; Wang, H. Inorganic particle enhanced polymer hollow fiber membranes with high mechanical properties. Mater Chem Phys. 2015, 167, 209–218. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purkait, M.K. Increase in hydrophilicity of polysulfone membrane using polyethylene glycol methyl ether. J. Membr. Sci. 2013, 437, 7–16. [Google Scholar] [CrossRef]
- Yang, Y.; Nie, C.; Deng, Y.; Cheng, C.; He, C.; Ma, L.; Zhao, C. Improving antifouling and antimicrobial efficiency of ultrafiltration membranes with functional carbon nanotubes. RSC Adv. 2016, 6, 88265–88276. [Google Scholar] [CrossRef]
- Nguyen, H.T.V.; Ngo, T.H.A.; Do, K.D.; Nguyen, M.N.; Dang, N.T.T.; Nguyen, T.T.H.; Vien, V.; Vu, T.A. Preparation and characterization of a hydrophilic membrane using graphene oxide. J. Chem. 2019, 2019, 3164373. [Google Scholar] [CrossRef] [Green Version]










| Membrane | EWC (%) | Porosity (%) |
|---|---|---|
| 0% CNT | 61.58 | 55.39 ± 5.6 |
| 0.5% p-CNT | 46.81 | 33.67 ± 4.8 |
| 1% p-CNT | 60.17 | 57.39 ± 2.6 |
| 1,5% p-CNT | 63.78 | 58.07 ± 4.2 |
| 1% fCNT | 68.36 | 60.89 ± 4.7 |
| Membrane | Mean Surface Roughness (Ra-nm) | Root Mean Surface Roughness (Rq-nm) |
|---|---|---|
| 0% CNT | 7.160 ± 1.5 | 9.010 ± 1,6 |
| 0.5% p-CNT | 3.523 ± 2.1 | 4.594 ± 2.8 |
| 1% p-CNT | 3.402 ± 1.9 | 4.315 ± 2.6 |
| 1,5% p-CNT | 2.736 ± 2.8 | 3.572 ± 1.2 |
| 1% fCNT | 1.865 ± 0.7 | 2.420 ± 1.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rameetse, M.S.; Aberefa, O.; Daramola, M.O. Effect of Loading and Functionalization of Carbon Nanotube on the Performance of Blended Polysulfone/Polyethersulfone Membrane during Treatment of Wastewater Containing Phenol and Benzene. Membranes 2020, 10, 54. https://doi.org/10.3390/membranes10030054
Rameetse MS, Aberefa O, Daramola MO. Effect of Loading and Functionalization of Carbon Nanotube on the Performance of Blended Polysulfone/Polyethersulfone Membrane during Treatment of Wastewater Containing Phenol and Benzene. Membranes. 2020; 10(3):54. https://doi.org/10.3390/membranes10030054
Chicago/Turabian StyleRameetse, Mabusha S., Oluseyi Aberefa, and Michael O. Daramola. 2020. "Effect of Loading and Functionalization of Carbon Nanotube on the Performance of Blended Polysulfone/Polyethersulfone Membrane during Treatment of Wastewater Containing Phenol and Benzene" Membranes 10, no. 3: 54. https://doi.org/10.3390/membranes10030054
APA StyleRameetse, M. S., Aberefa, O., & Daramola, M. O. (2020). Effect of Loading and Functionalization of Carbon Nanotube on the Performance of Blended Polysulfone/Polyethersulfone Membrane during Treatment of Wastewater Containing Phenol and Benzene. Membranes, 10(3), 54. https://doi.org/10.3390/membranes10030054