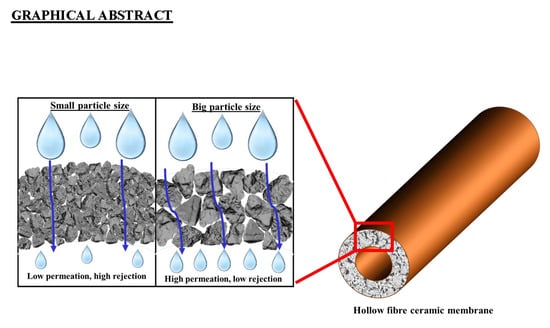
Influence of the Natural Zeolite Particle Size Toward the Ammonia Adsorption Activity in Ceramic Hollow Fiber Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Membrane Fabrication and Characterisation
2.3. Ammonia Removal by Adsorptive Natural Zeolite HFCM
3. Results and Discussion
3.1. Natural Zeolite HFCM Fabrication and Characterisation
3.1.1. Rheology of the Ceramic Suspension
3.1.2. Morphological Behavior of the HFCM
3.1.3. Crystallinity Properties of the HFCM
3.1.4. Mechanical Strength and Water Permeability of the HFCM
3.2. Ammonia Removal by Natural Zeolite HFCM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renge, V.; Khedkar, S.; Pande, S.V. Removal of heavy metals from wastewater using low cost adsorbents: A review. Sci. Rev. Chem. Commun. 2012, 2, 580–584. [Google Scholar]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 337–346. [Google Scholar]
- Atkins, P.F., Jr.; Scherger, D.A. A review of physical-chemical methods for nitrogen removal from wastewater. In Proceedings of the Conference on Nitrogen as a Water Pollutant; Elsevier: Pergamon, Turkey, 2013; pp. 713–719. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Q.; Xu, Y.; Luo, W. Corrosion behavior of bulk nanocrystalline copper in ammonia solution. Mater. Lett. 2011, 65, 857–859. [Google Scholar] [CrossRef]
- Qiu, W.; Kosuri, M.; Zhou, F.; Koros, W.J. Dehydration of ethanol–water mixtures using asymmetric hollow fiber membranes from commercial polyimides. J. Membr. Sci. 2009, 327, 96–103. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Ahmed, S.I.; Souaya, E.R.; Badawy, M.I.; Ulbricht, M. Fabrication of modified polyethersulfone membranes for wastewater treatment by submerged membrane bioreactor. Sep. Purif. Technol. 2017, 175, 36–46. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Jeong, S.; Lee, E.-J.; Tabatabai, S.A.A.; Leiknes, T. High flux and antifouling properties of negatively charged membrane for dyeing wastewater treatment by membrane distillation. Water Res. 2016, 103, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, L.; Wang, R.; Li, K.; Fane, A.G. Fabrication of novel poly (amide–imide) forward osmosis hollow fiber membranes with a positively charged nanofiltration-like selective layer. J. Membr. Sci. 2011, 369, 196–205. [Google Scholar] [CrossRef]
- Srijaroonrat, P.; Julien, E.; Aurelle, Y. Unstable secondary oil/water emulsion treatment using ultrafiltration: Fouling control by backflushing. J. Membr. Sci. 1999, 159, 11–20. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, X.; Liu, H.; Liu, S.; Yeung, K.L. Preparation and application of zeolite/ceramic microfiltration membranes for treatment of oil contaminated water. J. Membr. Sci. 2008, 325, 420–426. [Google Scholar] [CrossRef]
- Guizard, C.; Ayral, A.; Julbe, A. Potentiality of organic solvents filtration with ceramic membranes. A comparison with polymer membranes. Desalination 2002, 147, 275–280. [Google Scholar] [CrossRef]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Masmoudi, S.; Amar, R.B.; Larbot, A.; El Feki, H.; Salah, A.B.; Cot, L. Elaboration of inorganic microfiltration membranes with hydroxyapatite applied to the treatment of wastewater from sea product industry. J. Membr. Sci. 2005, 247, 1–9. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Rodriguez, M.A.; Srasra, E. Preparation of macroporous membrane using natural Kaolin and Tunisian lignite as a pore-forming agent. Desalin. Water Treat. 2016, 57, 13388–13393. [Google Scholar] [CrossRef]
- Fang, J.; Qin, G.; Wei, W.; Zhao, X. Preparation and characterization of tubular supported ceramic microfiltration membranes from fly ash. Sep. Purif. Technol. 2011, 80, 585–591. [Google Scholar] [CrossRef]
- Bai, C.-Y.; Li, Y.; Liu, Z.-M.; Liu, P.-W.; Deng, X.-Y.; Li, J.-B.; Yang, J. Fabrication and properties of mullite-bonded porous SiC membrane supports using bauxite as aluminum source. Ceram. Int. 2015, 41, 4391–4400. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef]
- Kouvelos, E.; Kesore, K.; Steriotis, T.; Grigoropoulou, H.; Bouloubasi, D.; Theophilou, N.; Tzintzos, S.; Kanelopoulos, N. High pressure N2/CH4 adsorption measurements in clinoptilolites. Microporous Mesoporous Mater. 2007, 99, 106–111. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Zhang, X.; Yang, J.; Liu, X.; Meng, G. Fabrication and characterization of low cost tubular mineral-based ceramic membranes for micro-filtration from natural zeolite. J. Membr. Sci. 2006, 281, 592–599. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Jia, Y. Membrane Technology: Past, Present and Future. In Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–45. [Google Scholar] [CrossRef]
- Yeon, S.-H.; Lee, K.-S.; Sea, B.; Park, Y.-I.; Lee, K.-H. Application of pilot-scale membrane contactor hybrid system for removal of carbon dioxide from flue gas. J. Membr. Sci. 2005, 257, 156–160. [Google Scholar] [CrossRef]
- Nymeijer, D.; Visser, T.; Assen, R.; Wessling, M. Composite hollow fiber gas–liquid membrane contactors for olefin/paraffin separation. Sep. Purif. Technol. 2004, 37, 209–220. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Adam, M.R.; Salleh, N.M.; Othman, M.H.D.; Matsuura, T.; Ali, M.H.; Puteh, M.H.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. The adsorptive removal of chromium (VI) in aqueous solution by novel natural zeolite based hollow fibre ceramic membrane. J. Environ. Manag. 2018, 224, 252–262. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Ismail, A.F.; Gani, P. Effect of kaolin particle size and loading on the characteristics of kaolin ceramic support prepared via phase inversion technique. J. Asian Ceram. Soc. 2016, 4, 164–177. [Google Scholar] [CrossRef]
- Kumar, P.S.; Korving, L.; Keesman, K.J.; van Loosdrecht, M.C.; Witkamp, G.-J. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics. Chem. Eng. J. 2019, 358, 160–169. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Hubadillah, S.K.; Puteh, M.H.; Harun, Z.; Ismail, A.F. Evaluating the sintering temperature control towards the adsorptivity of ammonia onto the natural zeolite based hollow fibre ceramic membrane. Int. J. Eng. Trans. B Appl. 2018, 31, 1398–1405. [Google Scholar] [CrossRef]
- Zhao, T.; Li, S.-H.; Shen, L.; Wang, Y.; Yang, X.-Y. The sized controlled synthesis of MIL-101(Cr) with enhanced CO2 adsorption property. Inorg. Chem. Commun. 2018, 96, 47–51. [Google Scholar] [CrossRef]
- Aghaei, Z.; Naji, L.; Hadadi Asl, V.; Khanbabaei, G.; Dezhagah, F. The influence of fumed silica content and particle size in poly (amide 6-b-ethylene oxide) mixed matrix membranes for gas separation. Sep. Purif. Technol. 2018, 199, 47–56. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Zornoza, B.; Friebe, S.; Caro, J.; Cao, S.; Sabetghadam, A.; Seoane, B.; Gascon, J.; Kapteijn, F.; Le Guillouzer, C.; et al. Influence of ZIF-8 particle size in the performance of polybenzimidazole mixed matrix membranes for pre-combustion CO2 capture and its validation through interlaboratory test. J. Membr. Sci. 2016, 515, 45–53. [Google Scholar] [CrossRef]
- María Arsuaga, J.; Sotto, A.; del Rosario, G.; Martínez, A.; Molina, S.; Teli, S.B.; de Abajo, J. Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes. J. Membr. Sci. 2013, 428, 131–141. [Google Scholar] [CrossRef]
- Kingsbury, B.F.K.; Li, K. A morphological study of ceramic hollow fibre membranes. J. Membr. Sci. 2009, 328, 134–140. [Google Scholar] [CrossRef]
- Kingsbury, B.F.; Wu, Z.; Li, K. A morphological study of ceramic hollow fibre membranes: A perspective on multifunctional catalytic membrane reactors. Catal. Today 2010, 156, 306–315. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.; Dong, Y.; Dong, X.; Cerneaux, S.; Hampshire, S.; Cao, J.; Zhu, L.; Zhu, Z.; Liu, J. A low-cost alumina-mullite composite hollow fiber ceramic membrane fabricated via phase-inversion and sintering method. J. Eur. Ceram. Soc. 2016, 36, 2057–2066. [Google Scholar] [CrossRef]
- Li, L.Y.; Tazaki, K.; Lai, R.; Shiraki, K.; Asada, R.; Watanabe, H.; Chen, M. Treatment of acid rock drainage by clinoptilolite—Adsorptivity and structural stability for different pH environments. Appl. Clay Sci. 2008, 39, 1–9. [Google Scholar] [CrossRef]
- Moradi, M.; Karimzadeh, R.; Moosavi, E.S. Modified and ion exchanged clinoptilolite for the adsorptive removal of sulfur compounds in a model fuel: New adsorbents for desulfurization. Fuel 2018, 217, 467–477. [Google Scholar] [CrossRef]
- Jamalzadeh, Z.; Haghighi, M.; Asgari, N. Synthesis, physicochemical characterizations and catalytic performance of Pd/carbon-zeolite and Pd/carbon-CeO2 nanocatalysts used for total oxidation of xylene at low temperatures. Front. Environ. Sci. Eng. 2013, 7, 365–381. [Google Scholar] [CrossRef]
- Amereh, M.; Haghighi, M.; Estifaee, P. The potential use of HNO3-treated clinoptilolite in the preparation of Pt/CeO2-Clinoptilolite nanostructured catalyst used in toluene abatement from waste gas stream at low temperature. Arab. J. Chem. 2018, 11, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.C.; Lira, H.D.; Lima, R.D.; Freitas, N.L. Effect of Sintering Temperature on Membranes Manufactured with Clays for Textile Effluent Treatment. Adv. Mater. Sci. Eng. 2015, 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.F.; Jaafar, J.; Aziz, A.A. Preparation and characterization of self-cleaning alumina hollow fiber membrane using the phase inversion and sintering technique. Ceram. Int. 2016, 42, 12312–12322. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Iwamoto, Y.; Honda, S.; Dzahir, M.I.H.M.; Yusop, M.Z.M. Fabrication of low cost, green silica based ceramic hollow fibre membrane prepared from waste rice husk for water filtration application. Ceram. Int. 2018, 44, 10498–10509. [Google Scholar] [CrossRef]
- Othman, N.H.; Wu, Z.; Li, K. A micro-structured La0.6Sr0.4Co0.2Fe0.8O3−δ hollow fibre membrane reactor for oxidative coupling of methane. J. Membr. Sci. 2014, 468, 31–41. [Google Scholar] [CrossRef]
- Abdullah, N.; Rahman, M.A.; Dzarfan Othman, M.H.; Jaafar, J.; Aziz, A.A. Preparation, characterizations and performance evaluations of alumina hollow fiber membrane incorporated with UiO-66 particles for humic acid removal. J. Membr. Sci. 2018, 563, 162–174. [Google Scholar] [CrossRef]
- Honda, S.; Ogihara, Y.; Hashimoto, S.; Iwamoto, Y. Thermal Shock Properties of Porous Alumina for Support Carrier of Hydrogen Membrane Materials. Adv. Bioceram. Porous Ceram. III 2010, 31, 127–137. [Google Scholar] [CrossRef]
- Mohammadi, F.; Mohammadi, T. Optimal conditions of porous ceramic membrane synthesis based on alkali activated blast furnace slag using Taguchi method. Ceram. Int. 2017, 43, 14369–14379. [Google Scholar] [CrossRef]
- Foo, K.Y.; Lee, L.K.; Hameed, B.H. Preparation of tamarind fruit seed activated carbon by microwave heating for the adsorptive treatment of landfill leachate: A laboratory column evaluation. Bioresour. Technol. 2013, 133, 599–605. [Google Scholar] [CrossRef]
- Hu, Y.-N.; Wang, H.-Y.; Cao, G.-P.; Meng, C.; Yuan, W.-K. The adsorption of toluenediamine from the wastewater by activated carbon in batch and fixed bed systems. Desalination 2011, 279, 54–60. [Google Scholar] [CrossRef]
- Zhou, L.; Qu, Z.G.; Chen, L.; Tao, W.Q. Lattice Boltzmann simulation of gas–solid adsorption processes at pore scale level. J. Comput. Phys. 2015, 300, 800–813. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Abu Samah, R.; Puteh, M.H.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lo, K.V. Ammonia removal from composting leachate using zeolite. I. Characterization of the zeolite. J. Environ. Sci. Health Part A 2001, 36, 1671–1688. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Xu, Z.; Han, T.; Chuan, S.; Zhu, T. Ammonia removal from leachate solution using natural Chinese clinoptilolite. J. Hazard. Mater. 2006, 136, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Lin, F.; Pang, W.Q. Ammonium exchange in aqueous solution using Chinese natural clinoptilolite and modified zeolite. J. Hazard. Mater. 2007, 142, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Rožić, M.; Cerjan-Stefanović, Š.; Kurajica, S.; Vančina, V.; Hodžić, E. Ammoniacal nitrogen removal from water by treatment with clays and zeolites. Water Res. 2000, 34, 3675–3681. [Google Scholar] [CrossRef]
- Farkas, A.; Rozic, M.; Barbaric-Mikocevic, Z. Ammonium exchange in leakage waters of waste dumps using natural zeolite from the Krapina region, Croatia. J. Hazard. Mater. 2005, 117, 25–33. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.N.; Khorasani, Z.; Gorjiara, M. Ammonia Removal from Aqueous Solutions by Iranian Natural Zeolite. Sep. Sci. Technol. 2008, 43, 960–978. [Google Scholar] [CrossRef]
- Weatherley, L.R.; Miladinovic, N.D. Comparison of the ion exchange uptake of ammonium ion onto New Zealand clinoptilolite and mordenite. Water Res. 2004, 38, 4305–4312. [Google Scholar] [CrossRef]
- Karadag, D.; Koc, Y.; Turan, M.; Armagan, B. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard. Mater. 2006, 136, 604–609. [Google Scholar] [CrossRef]
- Guo, X.; Zeng, L.; Li, X.; Park, H.S. Removal of Ammonium from RO Permeate of Anaerobically Digested Wastewater by Natural Zeolite. Sep. Sci. Technol. 2007, 42, 3169–3185. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A low cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep. Purif. Technol. 2019, 214, 31–39. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, D. Novel clay-based nanofibrous membranes for effective oil/water emulsion separation. Ceram. Int. 2017, 43, 9465–9471. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M. Microfiltration of cationic dyes using nano-clay membranes. Ceram. Int. 2017, 43, 15146–15159. [Google Scholar] [CrossRef]
- Harabi, A.; Guechi, A.; Condom, S. Production of supports and filtration membranes from Algerian kaolin and limestone. Procedia Eng. 2012, 33, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Purkait, M.; Mohanty, K. Preparation and characterization of low-cost ceramic microfiltration membranes for the removal of chromate from aqueous solutions. Appl. Clay Sci. 2010, 47, 317–324. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, L.; Dong, Y.; Li, L.; Liu, J. Waste-to-Resource Strategy To Fabricate Highly Porous Whisker-Structured Mullite Ceramic Membrane for Simulated Oil-in-Water Emulsion Wastewater Treatment. ACS Sustain. Chem. Eng. 2016, 4, 2098–2106. [Google Scholar] [CrossRef]
- Harabi, A.; Boudaira, B.; Bouzerara, F.; Foughali, L.; Zenikheri, F.; Guechi, A.; Ghouil, B.; Condom, S. Porous ceramic supports for membranes prepared from kaolin (DD3) and calcite mixtures. Acta Phys. Pol. A 2015, 127, 1164–1166. [Google Scholar] [CrossRef]
- de Oliveira Henriques, J.D.; Pedrassani, M.W.; Klitzke, W.; Mariano, A.B.; Vargas, J.V.C.; Vieira, R.B. Thermal treatment of clay-based ceramic membranes for microfiltration of Acutodesmus obliquus. Appl. Clay Sci. 2017, 150, 217–224. [Google Scholar] [CrossRef]












| Material | Material Form | Initial Ammonia Concentration (mg/L) | Ammonia Removal (%) | Reference |
|---|---|---|---|---|
| Canadian clinoptilolite | Powder suspension | 100 | 65 | [53] |
| Chinese clinoptilolite | Powder suspension | 115 | 80 | [54] |
| Chinese Na-clinoptilolite | Powder suspension | 250 | 77.16 | [55] |
| Croatian clinoptilolite | Powder suspension | 100 | 61.1 | [56] |
| Croatian clinoptilolite | Powder suspension | 800 | 75 | [57] |
| Iranian clinoptilolite | Powder suspension | 100 | 68 | [58] |
| New Zealand clinoptilolite | Powder suspension | 40 | 85.09 | [59] |
| Turkish clinoptilolite | Powder suspension | 150 | 70 | [60] |
| USA clinoptilolite | Powder suspension | 250 | 56 | [61] |
| Natural zeolite clinoptilolite | Adsorptive membrane | 50 | 82.97 | This study |
| Material | Fabrication Method | Sintering Temperature (°C) | Application | Membrane Mechanism | Reference |
|---|---|---|---|---|---|
| Kaolin | Extrusion | 1200–1500 | Arsenic removal | Membrane distillation | [62] |
| Chinese clay | Paste casting | 400 | Oil-in-water separation | Filtration | [63] |
| Iranian clay | Pressing | 900 | Cationic dye removal | Adsorption Filtration + | [64] |
| Kaolin + Limestone | Extrusion | 800–1100 | Support layer | Filtration | [65] |
| Indian clay | Paste casting | 800–1000 | Chromate removal | Flocculation + Filtration | [66] |
| Fly ash | Extrusion | 1100–1500 | Support layer | Filtration | [20] |
| Fly ash + bauxite | Pressing | 1200–1500 | Oil-in-water separation | Filtration | [67] |
| Kaolin + Calcium carbonate | Extrusion | 1150–1300 | Support layer | Filtration | [68] |
| Brazilian clay | Pressing | 1050 | Microalgae removal | Filtration | [69] |
| Natural zeolite clinoptilolite | Phase inversion extrusion | 1050 | Ammonia removal | Adsorption + Filtration | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, M.R.; Othman, M.H.D.; Sheikh Abdul Kadir, S.H.; Mohd Sokri, M.N.; Tai, Z.S.; Iwamoto, Y.; Tanemura, M.; Honda, S.; Puteh, M.H.; Rahman, M.A.; et al. Influence of the Natural Zeolite Particle Size Toward the Ammonia Adsorption Activity in Ceramic Hollow Fiber Membrane. Membranes 2020, 10, 63. https://doi.org/10.3390/membranes10040063
Adam MR, Othman MHD, Sheikh Abdul Kadir SH, Mohd Sokri MN, Tai ZS, Iwamoto Y, Tanemura M, Honda S, Puteh MH, Rahman MA, et al. Influence of the Natural Zeolite Particle Size Toward the Ammonia Adsorption Activity in Ceramic Hollow Fiber Membrane. Membranes. 2020; 10(4):63. https://doi.org/10.3390/membranes10040063
Chicago/Turabian StyleAdam, Mohd Ridhwan, Mohd Hafiz Dzarfan Othman, Siti Hamimah Sheikh Abdul Kadir, Mohd Nazri Mohd Sokri, Zhong Sheng Tai, Yuji Iwamoto, Masaki Tanemura, Sawao Honda, Mohd Hafiz Puteh, Mukhlis A. Rahman, and et al. 2020. "Influence of the Natural Zeolite Particle Size Toward the Ammonia Adsorption Activity in Ceramic Hollow Fiber Membrane" Membranes 10, no. 4: 63. https://doi.org/10.3390/membranes10040063
APA StyleAdam, M. R., Othman, M. H. D., Sheikh Abdul Kadir, S. H., Mohd Sokri, M. N., Tai, Z. S., Iwamoto, Y., Tanemura, M., Honda, S., Puteh, M. H., Rahman, M. A., & Jaafar, J. (2020). Influence of the Natural Zeolite Particle Size Toward the Ammonia Adsorption Activity in Ceramic Hollow Fiber Membrane. Membranes, 10(4), 63. https://doi.org/10.3390/membranes10040063