Electrochemical Impedance Spectroscopy of Anion-Exchange Membrane AMX-Sb Fouled by Red Wine Components
Abstract
:1. Introduction
2. Experiment
2.1. Membranes and Solutions
2.2. Methods
2.2.1. Membrane Fouling Procedure
2.2.2. Visualization
2.2.3. Microbiological Analysis
2.2.4. Electrochemical Impedance Spectroscopy
3. Results and Discussion
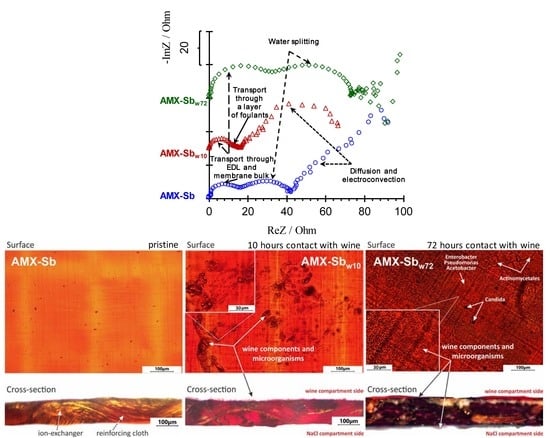
3.1. Optical Images
3.2. Results of Microbiological Analysis
3.3. Electrochemical Impedance Spectra
3.3.1. The High-Frequency EIS Domain
3.3.2. The Middle-Frequency EIS Domain
3.3.3. The Low-Frequency EIS Domain
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- El Rayess, Y.; Mietton-Peuchot, M. Membrane technologies in wine industry: An overview. Crit. Rev. Food Sci. Nutr. 2016, 56, 2005. [Google Scholar] [CrossRef]
- Romanov, A.M.; Zelentsov, V.I. Use of electrodialysis for the production of grape-based soft and alcoholic drinks. Surf. Eng. Appl. Elect. 2007, 43, 279–286. [Google Scholar] [CrossRef]
- Serre, E.; Rozoy, E.; Pedneault, K.; Lacour, S.; Bazinet, L. Deacidification of cranberry juice by electrodialysis: Impact of membrane types and configurations on acid migration and juice physicochemical characteristics. Sep. Purif. Technol. 2016, 163, 228–237. [Google Scholar] [CrossRef]
- De Pinho, M.N. Membrane processes in must and wine industries. In Membrane Technology; Peinemann, K.-V., Nunes, S.P., Giorno, L., Eds.; Wiley-VCH: Weinheim, Germany, 2010; Volume 3, pp. 105–118. [Google Scholar]
- Riponi, C.; Nauleau, F.; Amati, A.; Arfelli, G.; Castellari, M. Electrodialysis. 2. Tartrate stabilization of wines by electrodialysis. Rev. Fr. Oenol. 1992, 137, 59–63. [Google Scholar]
- Low, L.; O’Neill, B.; Ford, C.; Godden, J.; Gishen, M.; Colby, C. Economic evaluation of alternative technologies for tartrate stabilisation of wines. Int. J. Food Sci. Technol. 2008, 43, 1202–1216. [Google Scholar] [CrossRef]
- Gonçalves, F.; Fernandes, C.; dos Santos, P.C.; de Pinho, M.N. Wine tartaric stabilization by electrodialysis and its assessment by the saturation temperature. J. Food Eng. 2003, 59, 229–235. [Google Scholar] [CrossRef]
- Rozoy, E.; Boudesocque, L.; Bazinet, L. Deacidification of cranberry juice by electrodialysis with bipolar membranes. J. Agric. Food Chem. 2015, 63, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Academic Press: Cambridge, MA, USA, 2014; p. 751. [Google Scholar]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Sarapulova, V.; Nevakshenova, E.; Nebavskaya, X.; Kozmai, A.; Aleshkina, D.; Pourcelly, G.; Nikonenko, V.; Pismenskaya, N. Characterization of bulk and surface properties of anion-exchange membranes in initial stages of fouling by red wine. J. Membr. Sci. 2018, 559, 170–182. [Google Scholar] [CrossRef]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinet, L.; Dammak, L. Identification of phenolic compounds and their fouling mechanisms in ion-exchange membranes used at an industrial scale for wine tartaric stabilization by electrodialysis. Sep. Purif. Technol. 2020, 233, 115995. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Pourcelly, G.; Bazinet, L. How pulse modes affect proton-barriers and anion-exchange membrane mineral fouling during consecutive electrodialysis treatments. J. Colloid Interface Sci. 2013, 392, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, M.; Cui, Y.; Alali, A.; Atkinson, J.D. Phenol adsorption and desorption with physically and chemically tailored porous polymers: Mechanistic variability associated with hyper-cross-linking and amination. J. Hazard. Mater. 2019, 361, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, J.; Boschet, J.; Kammerer, D.R.; Carle, R. Enrichment and fractionation of major apple flavonoids, phenolic acids and dihydrochalcones using anion exchange resins. LWT Food Sci. Technol. 2011, 44, 1079–1087. [Google Scholar] [CrossRef]
- Caetano, M.; Valderrama, C.; Farran, A.; Cortina, J.L. Phenol removal from aqueous solution by adsorption and ion exchange mechanisms onto polymeric resins. J. Colloid Interface Sci. 2009, 338, 402–409. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, S.-T. Effect of pH on fumaric acid adsorption onto IRA900 ion exchange resin. Sep. Sci. Technol. 2015, 50, 56–63. [Google Scholar] [CrossRef]
- Hashim, H.; Wan Ahmad, W.Y.; Zubairi, S.I.; Maskat, M.Y. Effect of pH on adsorption of organic acids and phenolic compounds by amberlite IRA 67 resin. J. Teknol. 2019, 81, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine, Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; p. 450. [Google Scholar]
- Ping, Q.; Cohen, B.; Dosoretz, C.; He, Z. Long-term investigation of fouling of cation and anion exchange membranes in microbial desalination cells. Desalination 2013, 325, 48–55. [Google Scholar] [CrossRef]
- Drews, A. Membrane fouling in membrane bioreactors-characterisation, contradictions, cause and cures. J. Membr. Sci. 2010, 363. [Google Scholar] [CrossRef]
- Baker, J.S.; Dudley, L.Y. Biofouling in membrane systems—A review. Desalination 1998, 118, 81–89. [Google Scholar] [CrossRef]
- Wingender, J.; Flemming, H.C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Multon, J.L.; Flanzy, C. Oenologie: Fondements Scientifiques et Technologiques; TEC&DOC Lavoisier: Paris, France, 1998. [Google Scholar]
- Park, J.S.; Chilcott, T.C.; Coster, H.G.L.; Moon, S.H. Characterization of BSA-fouling of ion-exchange membrane systems using a subtraction technique for lumped data. J. Membr. Sci. 2005, 246, 137–144. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, J.H.; Yeon, K.H.; Moon, S.H. An approach to fouling characterization of an ion-exchange membrane using current-voltage relation and electrical impedance spectroscopy. J. Colloid Interface Sci. 2006, 294, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Merino-Garcia, I.; Kotoka, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Characterization of poly(Acrylic) acid-modified heterogenous anion exchange membranes with improved monovalent permselectivity for RED. Membranes 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, S.; Cao, H.; Xu, B.; Zhao, Z.; Cao, R.; Chang, J.; Duan, F.; Wen, H. Anion exchange nanocomposite membranes modified with graphene oxide and polydopamine: Interfacial structure and antifouling applications. ACS Appl. Nano Mater. 2020, 3, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Pintossi, D.; Saakes, M.; Borneman, Z.; Nijmeijer, K. Electrochemical impedance spectroscopy of a reverse electrodialysis stack: A new approach to monitoring fouling and cleaning. J. Power Sources 2019, 444, 227302. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, H.; Wang, J.; Wen, H.; Li, J. Characterization of fouling and concentration polarization in ion exchange membrane by in-situ electrochemical impedance spectroscopy. J. Membr. Sci. 2020, 594, 117443. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, S.; Cao, H.; Li, Y. Electrochemical impedance spectroscopy and surface properties characterization of anion-exchange membrane fouled by sodium dodecyl sulfate. J. Membr. Sci. 2017, 530, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, H.D.; Dibiani, R. Experimental and theoretical investigations of steady and transient states in systems of ion exchange bipolar membranes. J. Membr. Sci. 2004, 228, 17–43. [Google Scholar] [CrossRef]
- Zabolotskii, V.; Sheldeshov, N.; Melnikov, S. Heterogeneous bipolar membranes and their application in electrodialysis. Desalination 2014, 342, 183–203. [Google Scholar] [CrossRef]
- Kniaginicheva, E.; Pismenskaya, N.; Melnikov, S.; Belashova, E.; Sistat, P.; Cretin, M.; Nikonenko, V. Water splitting at an anion-exchange membrane as studied by impedance spectroscopy. J. Membr. Sci. 2015, 496, 78–83. [Google Scholar] [CrossRef]
- Sistat, P.; Kozmai, A.; Pismenskaya, N.; Larchet, C.; Pourcelly, G.; Nikonenko, V. Low-frequency impedance of an ion-exchange membrane system. Electrochim. Acta 2008, 53, 6380. [Google Scholar] [CrossRef]
- Kozmai, A.E.; Nikonenko, V.V.; Pismenskaya, N.D.; Mareev, S.A.; Belova, E.I.; Sistat, P. Use of electrochemical impedance spectroscopy for determining the diffusion layer thickness at the surface of ion-exchange membranes. Petrol. Chem. 2012, 52, 614. [Google Scholar] [CrossRef]
- Astom Corporation. Available online: http://www.astom-corp.jp/en/product/10.html (accessed on 14 December 2020).
- Le, X.T. Permselectivity and microstructure of anion exchange membranes. J. Colloid Interface Sci. 2008, 325, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs. structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Calull, M.; Marce, R.M.; Borrull, F. Determination of carboxylic acids, sugars, glycerol and ethanol in wine and grape must by ion-exchange high-performance liquid chromatography with refractive index detection. J. Chromatogr. A 1992, 590, 215–222. [Google Scholar] [CrossRef]
- Hussain, Z.; Ali, A.; Khan, K.M.; Perveen, S.; Mabood, F. A novel Spectrophotometric method for the trace analysis of glucose. J. Pharm. Res. 2011, 4, 4731–4733. [Google Scholar]
- Harrigan, W.F.; McCance, M.E. Laboratory Methods in Microbiology; Academic press: Cambridge, MA, USA, 2014; p. 374. [Google Scholar]
- Belashova, E.D.; Melnik, N.A.; Pismenskaya, N.D.; Shevtsova, K.A.; Nebavsky, A.V.; Lebedev, K.A.; Nikonenko, V.V. Overlimiting mass transfer through cation-exchange membranes modified by Nafion film and carbon nanotubes. Electrochim. Acta 2012, 59, 412. [Google Scholar] [CrossRef]
- Newman, J.S. Electrochemical Systems; Prentice Hall: Englewood Cliffs, NJ, USA, 1973; p. 309. [Google Scholar]
- Barsukov, Y.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; Wiley: New York, NY, USA, 2005; p. 616. [Google Scholar]
- Nikonenko, V.; Kozmai, A. Electrical equivalent circuit of an ion-exchange membrane system. Electrochim. Acta 2011, 56, 1262–1269. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014; p. 369. [Google Scholar]
- Simoes Costa, A.M.; Costa Sobral, M.M.; Delgadillo, I.; Cerdeira, A.; Rudnitskaya, A. Astringency quantification in wine: Comparison of the electronic tongue and FT-MIR spectroscopy. Sens. Actuators B Chem. 2015, 207, 1095–1103. [Google Scholar] [CrossRef]
- Luong, J.H.T. Kinetics of ethanol inhibition in alcohol fermentation. Biotechnol. Bioeng. 1985, 27, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.; Ezeji, T.C.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Dammak, L.; Larchet, C.; Grande, D. Ageing of ion-exchange membranes in oxidant solutions. Sep. Purif. Technol. 2009, 69, 43–47. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B.; Futerman, A.; Gitis, V.; Nikonenko, V. Reexamination of electrodiffusion time scales. Phys. Rev. E 2009, 79, 021506. [Google Scholar] [CrossRef]
- Abdu, S.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-layer modification of cation-exchange membranes controls ion selectivity and water splitting. ACS Appl. Mater. Inter. 2014, 6, 1843. [Google Scholar] [CrossRef]
- Długołęcki, P.; Ogonowski, P.; Metz, S.J.; Saakes, M.; Nijmeijer, K.; Wessling, M. On the resistances of membrane, diffusion boundary layer and double layer in ion-exchange membrane transport. J. Membr. Sci. 2010, 349, 369–379. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Wiley-Interscience: New York, NY, USA, 1987. [Google Scholar]
- Femmer, R.; Marti-Calatayud, M.C.; Wessling, M. Mechanistic modeling of the dielectric impedance of layered membrane architectures. J. Membr. Sci. 2016, 520, 29–36. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Nikonenko, V.; Auclair, B.; Pourcelly, G. Transport of weak-electrolyte anions through anion-exchange membranes. Current-voltage characteristics. J. Membr. Sci. 2001, 189, 129–140. [Google Scholar] [CrossRef]
- Choi, J.-H.; Moon, S.-H. Structural change of ion-exchange membrane surfaces under high electric field and its effect on membrane properties. J. Colloid Interface Sci. 2003, 265, 93–100. [Google Scholar] [CrossRef]
- Tadros, T. Encyclopedia of Colloid and Interface Science; Springer: Berlin, Germany, 2013; p. 1449. [Google Scholar]
- Piazza, R. Protein interactions and association: An open challenge for colloid science. Curr. Opin. Colloid Interface Sci. 2004, 8, 515–522. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling phenomena on anion exchange membranes under the reverse electrodialysis process. J. Membr. Sci. 2017, 530, 232–239. [Google Scholar] [CrossRef]
- Strahl, H.; Errington, J. Bacterial membranes: Structure, domains, and function. Annu. Rev. Microbiol. 2017, 71, 519–538. [Google Scholar] [CrossRef]
- Herzberg, M.; Pandit, S.; Mauter, M.S.; Oren, Y. Bacterial biofilm formation on ion exchange membranes. J. Membr. Sci. 2020, 596, 117564. [Google Scholar] [CrossRef]
- Slouka, Z.; Senapati, S.; Yan, Y.; Chang, H.-C. Charge inversion, water splitting, and vortex suppression due to DNA sorption on ion-selective membranes and their ion-current signatures. Langmuir 2013, 29, 8275–8283. [Google Scholar] [CrossRef]
- Belloň, T.; Polezhaev, P.; Vobecká, L.; Slouka, Z. Fouling of a heterogeneous anion-exchange membrane and single anion-exchange resin particle by ssDNA manifests differently. J. Membr. Sci. 2019, 572, 619–631. [Google Scholar] [CrossRef]
- Simons, R. Water splitting in ion exchange membranes. Electrochim. Acta 1985, 30, 275–282. [Google Scholar] [CrossRef]











| Type | Homogeneous, Strong Base a |
|---|---|
| Thickness in 0.02 M NaCl solution, μm | 160 ± 10 b |
| Conductivity in 0.02 M NaCl solution, S m−1 | 0.28 ± 0.02 b |
| Ion-exchange capacity, meq g−1 (swollen membrane) | 1.30 ± 0.05 b,c |
| Water content, g H2O• (g dry membrane)−1 | 0.20 ± 0.05 b |
| Membrane density, g cm−3 | 1.10 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozmai, A.; Sarapulova, V.; Sharafan, M.; Melkonian, K.; Rusinova, T.; Kozmai, Y.; Pismenskaya, N.; Dammak, L.; Nikonenko, V. Electrochemical Impedance Spectroscopy of Anion-Exchange Membrane AMX-Sb Fouled by Red Wine Components. Membranes 2021, 11, 2. https://doi.org/10.3390/membranes11010002
Kozmai A, Sarapulova V, Sharafan M, Melkonian K, Rusinova T, Kozmai Y, Pismenskaya N, Dammak L, Nikonenko V. Electrochemical Impedance Spectroscopy of Anion-Exchange Membrane AMX-Sb Fouled by Red Wine Components. Membranes. 2021; 11(1):2. https://doi.org/10.3390/membranes11010002
Chicago/Turabian StyleKozmai, Anton, Veronika Sarapulova, Mikhail Sharafan, Karina Melkonian, Tatiana Rusinova, Yana Kozmai, Natalia Pismenskaya, Lasaad Dammak, and Victor Nikonenko. 2021. "Electrochemical Impedance Spectroscopy of Anion-Exchange Membrane AMX-Sb Fouled by Red Wine Components" Membranes 11, no. 1: 2. https://doi.org/10.3390/membranes11010002
APA StyleKozmai, A., Sarapulova, V., Sharafan, M., Melkonian, K., Rusinova, T., Kozmai, Y., Pismenskaya, N., Dammak, L., & Nikonenko, V. (2021). Electrochemical Impedance Spectroscopy of Anion-Exchange Membrane AMX-Sb Fouled by Red Wine Components. Membranes, 11(1), 2. https://doi.org/10.3390/membranes11010002