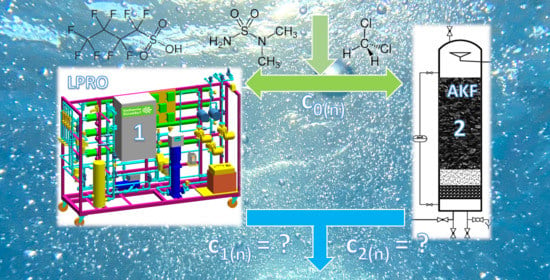
Removal of Trace Organic Contaminants by Parallel Operation of Reverse Osmosis and Granular Activated Carbon for Drinking Water Treatment
Abstract
:1. Introduction
- Preferential removal of nonpolar/hydrophobic substances;
- Competitive adsorption of TrOCs and background NOM to the activated carbon;
- Continuous decrease in activated carbon adsorption capacity with progression of operation time;
- Decreasing detention time with increasing filter velocity or reducing empty bed contact time.
2. Materials and Methods
2.1. Trace Organic Contaminants (TrOCs)
2.2. Membrane
2.3. Raw Water
2.4. Pilot Plant
2.5. Preparation of Stock Solutions
2.6. Membrane Autopsy
3. Results and Discussion
3.1. Performance Measurements during LPRO Operation
3.2. Membranes Autopsy
3.3. Removal Efficiency of TrOCs
3.3.1. Rejection of TrOCs via LPRO Operation
3.3.2. Removal of TrOCs via ACF Process
3.4. Evaluation of the Internal Plant Concentration Profiles
3.4.1. LPRO Operation
3.4.2. ACF
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bundesamt, S. Öffentliche Wasserversorgung und Öffentliche Abwasserentsorgung 2016; Statistisches Bundesamt: Wiesbaden, Germany, 2018. [Google Scholar]
- Kuhlmann, B.; Skark, C.; Zullei-Seibert, N. Sachverständigengutachten: Definition und Bewertung von Trinkwasserrelevanten Chemikalien im Rahmen der REACHVerordnung und Empfehlungen zum Screening nach Potentiell Kritischen Substanzen; Institut für Wasserforschung GmbH Dortmund Schwerte: Dortmund, Germany, 2010; p. 57. [Google Scholar]
- Berger, U.; Ost, N.; Sättler, D.; Schliebner, I.; Kühne, R.; Schüürmann, G.; Neumann, M.; Reemtsma, T. Assessment of Persistence, Mobility and Toxicity (PMT) of 167 REACH Registered Substances; Umweltbundesamt: Berlin, Germany, 2018. [Google Scholar]
- Hung, Y.T.; Lo, H.H.; Wang, L.K.; Taricska, J.R.; Li, K.H. (Eds.) Granular Activated Carbon Adsorption in Physicochemical Treatment Processes; Humana Press Inc.: Totowa, NJ, USA, 2005; Volume 3, pp. 573–633. [Google Scholar]
- Hobby, R.; Gimbel, R. Adsorption. In Wasseraufbereitung–Grundlagen und Verfahren, Oldenbourg Industrieverlag; Gimbel, R., Jekel, M., Ließfeld, R., Eds.; Oldenbourg Industrieverlag: München, Germany; Wien, Austria, 2004. [Google Scholar]
- Worch, E. Adsorption Technology in Water Treatment, Fundamentals, Processes, and Modeling; De Gruyter-Verlag: Berlin, Germany; Boston, MA, USA, 2012. [Google Scholar]
- Yangali-Quintanilla, V.A. Rejection of Emerging Organic Contaminants by Nanofiltration and Reverse Osmosis Membranes: Effects of Fouling, Modelling and Water Reuse; Delft University of Technology and UNESCO-IHE Institute for Water Education: Delft, The Netherlands, 2012. [Google Scholar]
- Yangali-Quintanilla, V.A.; Kim, T.-U.; Kennedy, M.; Amy, G. Modeling of RO/NF membrane rejections of PhACs and organic compounds: A statistical analysis. Drink. Water Eng. Sci. 2008, 1, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Nghiem, L.; Fujioka, T. Removal of Emerging Contaminants for Water Reuse by Membrane Technology in Emerging Membrane Technology for Sustainable Water Treatment; Singh, R., Hankins, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Plakas, K.V.; Karabelas, A.J. Removal of pesticides from water by NF and RO membranes—A review. Desalination 2012, 287, 255–265. [Google Scholar] [CrossRef]
- Verliefde, A.R.D.; Heijman, S.G.J.; Cornelissen, E.R. Rejection of trace organic pollutants with high pressure membranes (NF/RO). Environ. Prog. 2008, 27, 180. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Sadamani, A.; McConville, M. Modelling of pharmaceutically active compounds and endocrine disrupting compounds by clean and fouled nanofiltration membranes. Water Res. 2009, 43, 2349. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Process Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Schönsee, C.D.; Bucheli, T.D. Experimental determination of octanol–water partition coefficients of selected natural toxins. J. Chem. Eng. Data 2020, 65, 1946–1953. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, G.; Buonora, S.; Knoell, T.; Phipps, D.; Ridgway, H. Rejection of Pharmaceuticals by Reverse Osmosis Membranes: Quantitative Structure Activity Relationship (QSAR) Analysis; Final Report. 2004. Available online: https://www.semanticscholar.org/paper/Rejection-of-Pharmaceuticals-by-Reverse-Osmosis-Rodr%C3%ADguez-Buonora/06faa968479b777f15d2ff8cf9db7748bab35a78 (accessed on 24 November 2020).
- Wols, B.A.; Vries, D. On a QSAR approach for the prediction of priority compound degradation by water treatment processes. Water Sci. Technol. 2012, 66, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Braeken, L.; Bettens, B.; Boussu, K.; van der Meeren, P.; Cocquyt, J.; Vermant, J.; Van der Bruggen, B. Transport mechanisms of dissolved organic compounds in aqueous solution during nanofiltration. J. Membr. Sci. 2006, 279, 311–319. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Bellona, C.; Amy, G.; Kim, T.-U.; Adam, M.; Heberer, T. Rejection of emerging organic micropollutants in nanofiltration-reverse osmosis membrane applications. Water Environ. Res. 2005, 77, 40–48. [Google Scholar] [CrossRef]
- Tahaikta, M.; el Habbania, R.; Haddou, A.A.; Achary, I.; Amor, Z.; Taky, M.; Alami, A.; Boughriba, A.; Hafsi, M.; Elmidaoui, A. Fluoride removal from groundwater by nanofiltration. Desalination 2007, 212, 46–53. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory-design and description. J. Comput. Aided Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Toray. Ultra Low Pressure BWRO Elements, TMH10A; Toray Membrane Europe AG: Münchenstein, Switzerland, 2019. [Google Scholar]
- Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlamm-Untersuchung, 1st ed.; Updated 2020; Wiley-VCH: Weinheim, Germany, 1981.
- Vrouwenvelder, J.S.; Manolarakisa, S.A.; van der Hoek, J.P.; van Paassen, J.A.M.; van der Meere, W.G.J.; van Agtmaal, J.M.C.; Prummel, H.D.M.; Kruithof, J.C.; van Loosdrecht, M.C.M. Pilot-scale quantitative biofouling diagnosis in full scale nanofiltration and reverse osmosis installations. Water Res. 2008, 42, 4856–4868. [Google Scholar] [CrossRef] [PubMed]
- Norm. Water Analysis-Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC); EN 1484; BSI: Berlin, Germany, 1997. [Google Scholar]
- Norm. Water Quality-Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); EN ISO 11885; BSI: Berlin, Germany, 2009. [Google Scholar]
- Fujioka, T.; Oshima, N.; Suzuki, R.; Khan, S.J.; Roux, A.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. Rejection of small and uncharged chemicals of emerging concern by reverse osmosis membranes: The role of free volume space within the active skin layer. Sep. Purif. Technol. 2013, 116, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, T.; Oshima, N.; Suzuki, R.; Price, W.E.; Nghiem, L.D. Probing the internal structure of reverse osmosis membranes by positron annihilation spectroscopy: Gaining more insight into the transport of water and small solutes. J. Membr. Sci. 2015, 486, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Vrouwenvelder, J.S.; Picioreanu, C.; Kruithof, J.C.; van Loosdrecht, M.C.M. Biofouling in spiral wound membrane systems: Three-dimensional CFD model based evaluation of experimental data. J. Membr. Sci. 2010, 346, 71–85. [Google Scholar] [CrossRef]
- Haidari, A.H.; Heijman, S.G.J.; Van Der Meer, W.G.J. Optimal design of spacers in reverse osmosis. Sep. Purif. Technol. 2018, 192, 441–456. [Google Scholar] [CrossRef]
- Melin, T.; Rautenbach, R. Membranverfahren Grundlagen der Modul-und Anlagenauslegung; Springer: Berlin, Germany, 2007. [Google Scholar]
- Kegel, F.S.; Rietman, B.M.; Verliefde, A.R.D. Reverse osmosis followed by activated carbon filtration for efficient removal of organic micropollutants from river bank filtrate. Water Sci. Technol. 2010, 61, 2603–2610. [Google Scholar] [CrossRef]
- Samadi, M.T.; Nasseri, S.; Mesdaghinea, A.R.; Alizadehfard, R.A. Comparison of nanofitration efficiency with GAC adsorption and air stripping processes for CHCl3 removal from Teheran drinking water. J. Water Supply 2009, 58, 285–290. [Google Scholar] [CrossRef]
- Kazner, C. Advanced Wastewater Treatment by Nanofiltration and Activated Carbon for High Quality Water Reuse; RWTH Aachen University: Aachen, Germany, 2011. [Google Scholar]
- Bethmann, D.; Müller, U.; Baldauf, G. Spurenstoffentfernung aus weichen Brunnenwässern–Maximierung der Ausbeute und Minimierung der Einsatzmenge an Antiscalants beim Betrieb einer Umkehrosmoseanlage, Kurzfassung des Abschlussberichtes; DVGW-Technologiezentrum Wasser: Karlsruhe, Germany, 2012. [Google Scholar]
- Klüpfel, A. Nanofiltration bei der Aufbereitung von Trink-und Schwimmbeckenwasser–Foulingmechanismen und Rückhalt Anthropogener Kontaminanten; KIT: Karlsruhe, Germany, 2012. [Google Scholar]
- Gierszal, K.P.; Davis, J.G.; Hands, M.D.; Wilcox, D.S.; Slipchenko, L.V.; Ben-Amotz, D. π-Hydrogen Bonding in Liquid Water. J. Phys. Chem. Lett. 2011, 22, 2930–2933. [Google Scholar] [CrossRef]
- Mahlangu, T.O.; Schoutteten, K.V.K.M.; D’Haese, A.; van den Bussche, J.; Vanhaecke, L.; Thwala, J.M.; Mambae, B.B.; Verliefde, A.R.D. Role of permeate flux and specific membrane-foulant-solute affinity interactions (ΔGslm) in transport of trace organic solutes through fouled nanofiltration (NF) membranes. J. Membr. Sci. 2016, 518, 203–215. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Roux, A.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-nitrosamine rejection by nanofiltration and reverse osmosis membranes: The importance of membrane characteristics. Desalination 2013, 316, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Yamashita, N.; Nakada, N.; Tanaka, H. Removal characteristics of N-nitrosamines and their precursors by pilot-scale integrated membrane systems for water reuse. Int. J. Environ. Res. Public Health 2018, 15, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Nghiem, L.D. Nanofiltration of trace organic chemicals: A comparison between ceramic and polymeric membranes. Sep. Purif. Technol. 2014, 136, 258–264. [Google Scholar] [CrossRef]







| Compound 1 | Molecular Weight (g/mol) | LOG KOW 2 | pKa 3 | Charge at pH 7 4 |
|---|---|---|---|---|
| Group 1 | ||||
| Perfluorobutanoic acid (PFBA) | 214.05 | 2.25 | 0.08–0.4 | a |
| Perfluorohexane sulfonic acid (PFHxS) | 400.14 | 3.68 | 0.14 | a |
| Perfluorooctanoic acid (PFOA) | 414.09 | 4.93 | 1.3 | a |
| Perfluorobutane sulfonic acid (PFBS) | 300.12 | 2.34 | −3.13 | a |
| Perfluorohexanoic acid (PFHxA) | 314.07 | 3.59 | −0.16 | a |
| Perfluorooctansulfonic acid (PFOS) | 500.16 | 5.02 | 0.14 | a |
| Group 2 | ||||
| 1H-Benzotriazole (BTA) | 119.14 | 1.02 | 8.20 | n |
| Dimethylsufamide (DMS) | 124.19 | −1.16 | 10.4 | n |
| 5-Methyl benzotriazole (MBTA) | 133.15 | 1.4 | 8.66 | n |
| Metformin | 129.16 | −1.27 | 12.4 | c |
| Desphenylchloridazone (DPC) | 145.56 | −0.35 | 9.29 | n |
| Acesulfame | 163.17 | −0.34 | 2.0 | a |
| Caffeine | 194.19 | −0.10 | 10.4 | n |
| Isoproturon | 206.32 | 2.87 | 15.06 | n |
| Primidone | 218.28 | 0.91 | 12.3 | n |
| Terbutaline | 225.32 | 0.9 | 8.80 | c |
| Carbamazepine | 236.29 | 2.45 | 13.94 | n |
| Sulfamethoxazole | 253.31 | 0.89 | 5.60 | n |
| Propanolol | 259.34 | 2.98 | 9.46 | c |
| Diatrizoic acid (ATZ) | 613.93 | 1.75 | 0.92 | a |
| Group 3 | ||||
| N-Nitrosodimethylamine (NDMA) | 74.1 | −0.57 | 3.22 | n |
| Benzene | 78.12 | 2.13 | - | n |
| Dichloromethane (DCM) | 84.94 | 1.5 | - | n |
| Methyl tert-butyl ether (MTBE) | 88.17 | 0.94 | - | n |
| 1,2-Dichloroethane (DCE) | 98.97 | 1.48 | - | n |
| Ethyl tert-butyl ether (ETBE) | 102.2 | 1.43 | - | n |
| N-Nitrosomorpholine (NMOR) | 116.14 | −0.44 | 3.14 | n |
| Trichloromethane (TCM) | 119.38 | 1.97 | 15.5 | n |
| Trichloroethylene (TRI) | 131.39 | 2.61 | 25–27 | n |
| Tetrachloroethylene (TCE) | 165.83 | 3.40 | 14 | n |
| Ethylenediaminetetraacetic acid (EDTA) | 292.28 | −5.88 | 2.0; 2.7; 6.16; 10.26 | a |
| Pentetic acid (DTPA) | 393.4 | −8.53 | ~1.80 | a |
| Parameter 1 | Number of Analyses | Minimum | Maximum | Mean | Median |
|---|---|---|---|---|---|
| Temperature (°C) | 63 | 10.7 | 13.7 | 12.0 | 11.9 |
| pH | 32 | 7.12 | 7.49 | 7.31 | 7.30 |
| Oxygen (mg/L) | 11 | 3.0 | 3.9 | 2.7 | 2.8 |
| Electric conductivity (µS/cm) | 55 | 619 | 920 | 803 | 803 |
| Total acidity (mM) | 16 | 0.41 | 0.81 | 0.62 | 0.63 |
| Total alkalinity (mg/L CaCO3) | 13 | 183 | 226 | 203 | 201 |
| Calcium (mg/L) | 14 | 83.0 | 108 | 96.6 | 97.0 |
| Magnesium (mg/L) | 14 | 11.6 | 14.3 | 13.2 | 13.4 |
| Sodium (mg/L) | 14 | 36.7 | 61.2 | 48.4 | 48.7 |
| Potassium | 14 | 4.12 | 4.89 | 4.39 | 4.33 |
| Ammonia (mg/L) | 6 | 0.010 | 0.030 | 0.015 | 0.010 |
| Iron (mg/L) | 14 | 0.0075 | 0.11 | 0.015 | 0.0075 |
| Manganese (mg/L) | 14 | 0.0015 | 0.11 | 0.016 | 0.0015 |
| Nitrate (mg/L) | 25 | 14.9 | 33.3 | 21.8 | 21.5 |
| Silicon dioxide (mg/L) | 14 | 8.1 | 11.0 | 10.0 | 10.2 |
| Sulfate (mg/L) | 25 | 44.0 | 71.0 | 61.5 | 61.0 |
| Chloride (mg/L) | 24 | 69 | 121 | 92 | 89 |
| Fluoride (mg/L) | 25 | 0.11 | 0.17 | 0.15 | 0.15 |
| Bromide (mg/L) | 3 | 0.11 | 0.14 | 0.13 | 0.14 |
| Phosphate total (mg/L) | 12 | 0.044 | 0.060 | 0.051 | 0.050 |
| Total organic carbon (mg/L) | 5 | 0.55 | 0.79 | 0.64 | 0.64 |
| Total plate count at 20 °C (cfu/mL) | 63 | 0 | 6 | 0.33 | 0 |
| Total plate count at 36 °C (cfu/mL) | 63 | 0 | 3 | 0.14 | 0 |
| Treatment Step | Material |
|---|---|
| Anthracite sand filter (line 1/2) | 10 cm gravel, 30 mm and 80 cm silica sand, 0.71–1.25 mm; Euroquarz, Dorsten, Germany |
| 70 cm Hydroanthrazit; 1.4–2.5 mm (Euroquarz) | |
| GAC filter | 750 L Filtrasorb F-400, Chemviron Carbon GmbH, Beverungen, Germany, specific surface:1050–1200 m²/g, bulk density: 0.425 g/cm³ |
| LPRO | Eight 4-inch elements TMH 10A (Toray Membrane Europe AG [21]) of 8 m² each in four pressure vessels. |
| Antiscalant | Ropur RPI 2000A, Münchenstein, Switzerland and MT 4000, Grünbeck, Höchstädt a.d. Donau, Germany (active substance: phosphonic acid, DTPMP) with 0.2 g P/m³ in the feed |
| Group | Substance Class | Number of Substances | Spiking Concentration (µg/L) | Method of Analysis3 |
|---|---|---|---|---|
| 1 | Polyfluorinated aliphatic compounds 1 | 6 | 0.5 | HPLC/MS after SPE |
| 2 | Pharmaceuticals, pesticides and metabolites 2 | 14 | 1 | UPLC-MS/MS, direct injection |
| 3 | Volatile organic compounds (VOC) Nitrosamines Aminopolycarboxylic acids | 8 2 2 | 5 0.02 10 | SPME-GC/MS GC/MS after SPE GC/PND after liquid–liquid extraction |
| Parameter 1 | Effluent Anthracite Sand Filter 1 | Permeate | Reduction by LPRO (%) | Effluent Anthracite Sand Filter 1 | Permeate | Reduction by LPRO (%) |
|---|---|---|---|---|---|---|
| 25 L/m²·h | 31 L/m²·h | |||||
| Electric conductivity (µS/cm) | 713 | 16.7 | 97.7 | 704 | 14.6 | 97.9 |
| Calcium (mg/L) | 95 | 1.0 | 98.9 | 87 | <1 | >98.8 |
| Magnesium (mg/L) | 13.1 | 0.1 | 99.2 | 12.3 | <0.1 | >99.1 |
| Sodium (mg/L) | 45.9 | 2.4 | 94.8 | 35.5 | 1.80 | 94.9 |
| Potassium (mg/L) | 4.9 | 0.21 | 95.7 | 4.2 | 0.20 | 95.3 |
| Nitrate (mg/L) | 14.7 | 2.4 | 83.7 | 14.9 | 2.5 | 83.2 |
| Silicon dioxide (mg/L) | 13.29 | 0.43 | 96.8 | 11.6 | 0.18 | 98.4 |
| Sulfate (mg/L) | 55 | 3.9 | 92.9 | 55 | 1.0 | 99.1 |
| Chloride (mg/L) | 101 | 3.7 | 96.3 | 77 | 3.0 | 96.1 |
| Fluoride (mg/L) | 0.14 | <0.1 | >28.6 | 0.16 | <0.1 | >37.5 |
| Boron (mg/L) | 0.049 | 0.034 | 30.6 | 0.047 | 0.034 | 27.7 |
| Element Autopsy Position | Leading Module | Last Module | ||||
|---|---|---|---|---|---|---|
| (Front) | (Mid) | (End) | (Front) | (Mid) | (End) | |
| Calcium (µg/cm²) | 4.0 | 5.0 | 4.0 | 7.0 | 12 | 14 |
| Phosphorus total (µg/cm² PO4) | 3.0 | 3.7 | 2.6 | 6.8 | 12 | 15 |
| Silicon (µg/cm² SiO2) | 0.52 | 0.59 | 0.70 | 0.79 | 0.88 | 0.91 |
| Aluminum (µg/cm²) | 0.34 | 0.41 | 0.39 | 0.32 | 0.32 | 0.28 |
| Iron (µg/cm²) | 0.36 | 0.38 | 0.40 | 0.29 | 0.35 | 0.33 |
| Strontium (ng/cm²) | 60 | 80 | 98 | 90 | 119 | 103 |
| Barium (ng/cm²) | 30 | 37 | 32 | 39 | 53 | 57 |
| TOC (µg/cm²) | 33 | 41 | 30 | 29 | 27 | 27 |
| ATP (pg/cm²) | 560 | 550 | 500 | 250 | 280 | 300 |
| Specific loading for perfluoro compounds (m³/kg) | 1.1 | 6.2 | 13.9 | |||
| Specific loading for poly carboxylic acids and nitrosamines (m³/kg) | 3.7 | 9.4 | 17.9 | |||
| Specific loading for solid compounds (m³/kg) | 2.4 | 8.1 | 14.8–15.2 | |||
| Specific loading for volatiles (m³/kg) | 3.7 | 9.4 | 17.2–17.9 | |||
| Flux (L/m² h) | 25 | 25 | 31 | |||
| Sequence of experiments | 1 | 2 | 3 | 2 | 3 | 1 |
| Compound 1 | LPRO | ACF | ||||
| N-Nitrosodimethylamine (NDMA) | 21 | - | 35 | - | 60 | 70 |
| Benzene | 82/80 | 82 | - | >99 | - | >99/>99 |
| Dichloromethane (DCM) | 3.2/4.9 | 26 | 26 | 81 | 50 | 93/70 |
| Methyl tert-butyl ether (MTBE) | 99/>99 | >99 | >99 | >99 | 96 | 92/87 |
| 1,2-Dichloroethane (DCE) | 29/21 | 45 | 47 | >99 | >99 | >99/>99 |
| Ethyl tert-butyl ether (ETBE) | >99/>99 | >99 | >99 | >99 | >99 | >99/>99 |
| Acesulfame | >99/>99 | >99 | >99 | >99 | >99 | >99/>99 |
| N-Nitrosomorpholine (NMOR) | 95 | - | >91 | - | >96 | 98 |
| 1H-Benzotriazole (BTA) | 63/65 | 62 | 64 | 99 | >99 | >99/>99 |
| Trichloromethane (TCM) | 50/46 | 65 | 69 | >99 | >99 | >99/>99 |
| Dimethylsufamide (DMS) | >96/>96 | 95 | 97 | 85 | 15 | 8 /11 |
| Metformin | 90/91 | 96 | 96 | 82 | 63 | 97/87 |
| Trichloroethylene (TRI) | 52/48 | 67 | 61 | >99 | >99 | >99/99 |
| 5-Methyl benzotriazole (MBTA) | 80/82 | 82 | 79 | >99 | >99 | >99/>99 |
| Desphenylchloridazon (DPC) | 88/90 | 91 | 92 | >98 | >98 | 96/97 |
| Tetrachloroethylene (TCE) | 97/>96 | 91 | 90 | >98 | >95 | >98/>98 |
| Caffeine | >98/>98 | >98 | 95 | >99 | 97 | >99/>99 |
| Isoproturon | 90/>90 | >91 | - | >92 | - | >92/>91 |
| Perfluorbutanoic acid (PFBA) | >99 | >99 | >99 | >99 | >99 | 68 |
| Primidone | >98/>98 | >98 | >99 | >98 | >99 | >99/>99 |
| Terbutaline | >99/>99 | >98 | >97 | >97 | >99 | >99/>99 |
| Carbamazepine | >92/>92 | >93 | >99 | >93 | >99 | >93/>92 |
| Sulfamethoxazole | >98/>98 | >99 | >98 | >99 | >99 | >99/>99 |
| Propranolol | 85/>98 | >99 | >99 | >99 | >99 | 97/>99 |
| Ethylenediaminetetraacetic acid (EDTA) | 95 | >96 | - | >96 | 98 | 75 |
| Perfluorbutane sulfonic acid (PFBS) | >99 | >99 | >99 | >99 | >99 | >99 |
| Perfluorohexanoic acid (PFHxA) | >99 | >99 | >99 | >99 | >99 | >99 |
| Pentetic acid (DTPA) | 87 | 89 | 92 | 89 | 97 | >99 |
| Perfluorohexane sulfonic acid (PFHxS) | >99 | >99 | >99 | >99 | >99 | >99 |
| Perfluorooctanoic acid (PFOA) | >96 | >97 | >98 | >98 | >99 | >97 |
| Perfluorooctansulfonic acid (PFOS) | >95 | >97 | >99 | >97 | >99 | >96 |
| Diatrizoic acid (ATZ) | >98/>98 | >99 | >99 | >99 | >99 | >99/>99 |
| LPRO Process | ACFProcess | |
| Advantages |
|
|
| Drawbacks |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konradt, N.; Kuhlen, J.G.; Rohns, H.-P.; Schmitt, B.; Fischer, U.; Binder, T.; Schumacher, V.; Wagner, C.; Kamphausen, S.; Müller, U.; et al. Removal of Trace Organic Contaminants by Parallel Operation of Reverse Osmosis and Granular Activated Carbon for Drinking Water Treatment. Membranes 2021, 11, 33. https://doi.org/10.3390/membranes11010033
Konradt N, Kuhlen JG, Rohns H-P, Schmitt B, Fischer U, Binder T, Schumacher V, Wagner C, Kamphausen S, Müller U, et al. Removal of Trace Organic Contaminants by Parallel Operation of Reverse Osmosis and Granular Activated Carbon for Drinking Water Treatment. Membranes. 2021; 11(1):33. https://doi.org/10.3390/membranes11010033
Chicago/Turabian StyleKonradt, Norbert, Jan Gerrit Kuhlen, Hans-Peter Rohns, Birgitt Schmitt, Uwe Fischer, Timo Binder, Vera Schumacher, Christoph Wagner, Stefan Kamphausen, Uwe Müller, and et al. 2021. "Removal of Trace Organic Contaminants by Parallel Operation of Reverse Osmosis and Granular Activated Carbon for Drinking Water Treatment" Membranes 11, no. 1: 33. https://doi.org/10.3390/membranes11010033
APA StyleKonradt, N., Kuhlen, J. G., Rohns, H. -P., Schmitt, B., Fischer, U., Binder, T., Schumacher, V., Wagner, C., Kamphausen, S., Müller, U., Sacher, F., Janknecht, P., Hobby, R., ElSherbiny, I. M. A., & Panglisch, S. (2021). Removal of Trace Organic Contaminants by Parallel Operation of Reverse Osmosis and Granular Activated Carbon for Drinking Water Treatment. Membranes, 11(1), 33. https://doi.org/10.3390/membranes11010033