Membrane Distillation: Pre-Treatment Effects on Fouling Dynamics
Abstract
:1. Introduction
2. Materials and Methods
3. Mathematical Modeling
3.1. Model Prediction Capability
3.2. Assessment of Fouling Resistance
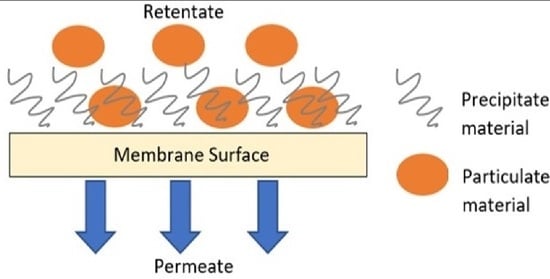
3.3. Dynamic of Fouling Deposition
4. Results and Discussion
4.1. Experimental Data and Fouling Resistance Assessment
4.2. Dynamics of Fouling Deposition
5. Implications for the Membrane Distillation Technology
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acquah, T.B.F.; Aristotelous, P.M.; Cox, W.; Fattah, Q.N.; Mejia, M.C.F.; Martinez, L.E.G.; Sadler, B.S.; Voropaev, G.V. The Role of Water in Socio-Economic Development. In Report 1 of IHP-II Project C1. Prepared for the International Hydrological Programme by the Working Group of Project C1 (IHP-II); Cox, W.E., Ed.; United Nations Educational, Scientific and Cultural Organization (UNESCO): Paris, France, 1987; pp. 46–91. [Google Scholar]
- Guelli Ulson de Souza, S.M.D.A.; Xavier, M.F.; da Silva, A.; Ulson de Souza, A.A. Water Reuse and Wastewater Minimization in Chemical Industries Using Differentiated Regeneration of Contaminants. Ind. Eng. Chem. Res. 2011, 50, 7428–7436. [Google Scholar] [CrossRef]
- Connor, R. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; United Nations Educational, Scientific and Cultural Organization (UNESCO): Paris, France, 2018; pp. 1–139. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000261424 (accessed on 16 November 2021).
- Stewart, B.; Buytaert, W.; Mishra, A.; Zandaryaa, S.; Connor, R.; Timmerman, J.; Uhlenbrook, S.; Hada, R. The State of Water Resources in the Context of Climate Change. In United Nations World Water Development Report 2020 Water Climate Change; Connor, R., Ed.; United Nations Educational, Scientific and Cultural Organization (UNESCO): Paris, France, 2020; pp. 1–29. [Google Scholar]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Eco-Efficient Process Improvement at the Early Development Stage: Identifying Environmental and Economic Process Hotspots for Synergetic Improvement Potential. Environ. Sci. Technol. 2018, 52, 5959–5967. [Google Scholar] [CrossRef]
- Barrington, D.; Prior, A.; Ho, G. The role of water auditing in achieving water conservation in the process industry. J. Clean. Prod. 2013, 52, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Santos, P.G.; Scherer, C.M.; Fisch, A.G.; Rodrigues, M.A.S. Petrochemical wastewater treatment: Water recovery using membrane distillation. J. Clean. Prod. 2020, 267, 121985. [Google Scholar] [CrossRef]
- Thomé, A.C.; Santos, P.G.; Fisch, A.G. Using rainwater in cooling towers: Design and performance analysis for a petrochemical company. J. Clean. Prod. 2019, 224, 275–283. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Choi, Y.; Vigneswaran, S. Membrane distillation for wastewater reverse osmosis concentrate treatment with water reuse potential. J. Membr. Sci. 2017, 524, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, M.M.A.; Kargari, A. A Review on Applications of Membrane Distillation (MD) Process for Wastewater Treatment. J. Membr. Sci. Res. 2015, 1, 101–112. [Google Scholar] [CrossRef]
- Thomas, N.; Mavukkandy, M.O.; Loutatidou, S.; Arafat, H.A. Membrane distillation research & implementation: Lessons from the past five decades. Sep. Purif. Technol. 2017, 189, 108–127. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; García-Payo, M.; Khayet, M.; El Kharraz, J.; Chaouch, M. Application of direct contact membrane distillation for textile wastewater treatment and fouling study. Sep. Purif. Technol. 2018, 209, 815–825. [Google Scholar] [CrossRef]
- Dow, N.; García, J.V.; Niadoo, L.; Milne, N.; Zhang, J.; Gray, S.; Duke, M. Demonstration of membrane distillation on textile waste water: Assessment of long term performance, membrane cleaning and waste heat integration. Environ. Sci. Water Res. Technol. 2017, 3, 433–449. [Google Scholar] [CrossRef]
- Osman, M.; Schoeman, J.; Baratta, L. Desalination/concentration of reverse osmosis and electrodialysis brines with membrane distillation. Desalin. Water Treat. 2010, 24, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Tufa, R.A.; Di Profio, G.; Fontananova, E.; Avci, A.H.; Curcio, E. Forward Osmosis, Reverse Electrodialysis and Membrane. Distillation 2019, 365–385. [Google Scholar] [CrossRef]
- Ren, M.; Ning, P.; Xu, J.; Qu, G.; Xie, R. Concentration and treatment of ceric ammonium nitrate wastewater by integrated electrodialysis-vacuum membrane distillation process. Chem. Eng. J. 2018, 351, 721–731. [Google Scholar] [CrossRef]
- Osman, M.S.; Masindi, V.; Abu-Mahfouz, A.M. Computational and experimental study for the desalination of petrochemical industrial effluents using direct contact membrane distillation. Appl. Water Sci. 2019, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Ricceri, F.; Giagnorio, M.; Farinelli, G.; Blandini, G.; Minella, M.; Vione, D.; Tiraferri, A. Desalination of Produced Water by Membrane Distillation: Effect of the Feed Components and of a Pre-treatment by Fenton Oxidation. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.Z.; Han, L.; Chow, W.H.; Fane, A.G.; Chew, J.W. Influence of module orientation and geometry in the membrane distillation of oily seawater. Desalination 2017, 423, 111–123. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.; Wang, R. Recent advances in membrane development for treating surfactant- and oil-containing feed streams via membrane distillation. Adv. Colloid Interface Sci. 2019, 273, 102022. [Google Scholar] [CrossRef]
- Carrero-Parreño, A.; Onishi, V.C.; Ruiz-Femenia, R.; Salcedo-Díaz, R.; Caballero, J.A.; Reyes-Labarta, J.A. Optimization of multistage membrane distillation system for treating shale gas produced water. Desalination 2019, 460, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Bush, J.; Vanneste, J.; Gustafson, E.M.; Waechter, C.A.; Jassby, D.; Turchi, C.S.; Cath, T.Y. Prevention and management of silica scaling in membrane distillation using pH adjustment. J. Membr. Sci. 2018, 554, 366–377. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Zhong, L.; Song, C.; Shi, W.; Cui, F.; Wang, W. Breathable and asymmetrically superwettable Janus membrane with robust oil-fouling resistance for durable membrane distillation. J. Membr. Sci. 2018, 563, 602–609. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Du, X.; Tong, T.; Cath, T.Y.; Lee, J. Antiwetting and Antifouling Janus Membrane for Desalination of Saline Oily Wastewater by Membrane Distillation. ACS Appl. Mater. Interfaces 2019, 11, 18456–18465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and wetting in the membrane distillation driven wastewater reclamation process—A review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef] [PubMed]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, J.H.V. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Lee, W.; Ng, Z.; Hubadillah, S.; Goh, P.; Lau, W.; Othman, M.; Ismail, A.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Qin, W.; Xie, Z.; Ng, D.; Ye, Y.; Ji, X.; Gray, S.; Zhang, J. Comparison of colloidal silica involved fouling behavior in three membrane distillation configurations using PTFE membrane. Water Res. 2017, 130, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Dyrda, K.M.; Wilke, V.; Haas-Santo, K.; Dittmeyer, R. Experimental Investigation of the Gas/Liquid Phase Separation Using a Membrane-Based Micro Contactor. ChemEngineering 2018, 2, 55. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, C.; Farris, K.; Kilduff, J. Membrane Fouling, Modelling and Recent Developments for Mitigation. In Emerging Membrane Technology Sustain Water Treatment; Hankins, N.P., Singh, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 433–462. [Google Scholar] [CrossRef]
- Karam, A.M.; Laleg-Kirati, T.M. Membrane fouling modeling and detection in direct contact membrane distillation. J. Process Control 2019, 81, 190–196. [Google Scholar] [CrossRef]
- Gryta, M. Polyphosphates used for membrane scaling inhibition during water desalination by membrane distillation. Desalination 2012, 285, 170–176. [Google Scholar] [CrossRef]
- Gryta, M. Alkaline scaling in the membrane distillation process. Desalination 2008, 228, 128–134. [Google Scholar] [CrossRef]
- Gryta, M. Effect of iron oxides scaling on the MD process performance. Desalination 2007, 216, 88–102. [Google Scholar] [CrossRef]
- Singh, R. Water and Membrane Treatment, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Bott, T.R. Fouling of Heat Exchangers, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Martynenko, O.G.; Pavlyukevich, N.V. Heat and mass transfer in porous media. J. Eng. Phys. Thermophys. 1998, 71, 1–13. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Ghajar, A.J. Heat and Mass Transfer Fundamentals and Applications, 4th ed.; McGraw-Hill: New York, NY, USA, 2011; p. 835. [Google Scholar]
- Koretsky, M.D. Engineering and Chemical Thermodynamics, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2012; p. 414. [Google Scholar]
- Curcio, E.; Drioli, E. Membrane Distillation and Related Operations—A Review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Nariyoshi, Y.N. Estudo dos Fundamentos de Cristalização Assistida por Destilação com Membranas em Aplicação de Dessalinização de Água. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, September 2016. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, S.O.; Camacho, L.M. Heat and Mass Transport in Modeling Membrane Distillation Configurations: A Review. Front. Energy Res. 2018, 6, 130. [Google Scholar] [CrossRef]
- Leitch, M.E.; Lowry, G.V.; Mauter, M.S. Characterizing convective heat transfer coefficients in membrane distillation cassettes. J. Membr. Sci. 2017, 538, 108–121. [Google Scholar] [CrossRef]
- Martínez-Díez, L.; Vázquez-González, M.I. Temperature and concentration polarization in membrane distillation of aqueous salt solutions. J. Membr. Sci. 1999, 156, 265–273. [Google Scholar] [CrossRef]
- Easteal, A.J.; Price, W.E.; Woolf, L.A. Diaphragm cell for high-temperature diffusion measurements. Tracer Diffusion coefficients for water to 363 K. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 1091–1097. [Google Scholar] [CrossRef]
- Jones, F.; Harris, G.L. ITS-90 density of water formulation for volumetric standards calibration. J. Res. Natl. Inst. Stand. Technol. 1992, 97, 335–340. [Google Scholar] [CrossRef]















| Parameter | |
|---|---|
| Supplier | Clarcor |
| Model | Aspire® QL217 |
| Type | Hydrophobic |
| Functional layer | PTFE |
| Support layer | PP fibers |
| Thickness (μm) | 150–250 |
| Nominal pore size (μm) | 0.2 |
| Porosity (%) | 80 |
| Water entry pressure (barg) | ≥1 |
| Contact angle (°) | 120 |
| Filtration area (m2) | 0.0132 |
| Parameter | Test 1 | Test 2 | Test 3 |
|---|---|---|---|
| Inlet temperature of retentate flow (°C) | 57.9 | 56.8 | 58.2 |
| Outlet temperature of retentate flow (°C) | 55.9 | 55.0 | 56.2 |
| Inlet temperature of permeate flow (°C) | 29.5 | 31.4 | 29.5 |
| Outlet temperature permeate flow (°C) | 31.7 | 33.7 | 31.6 |
| Pressure of retentate flow (barg) | 0.20 | 0.19 | 0.20 |
| Pressure of permeate flow (barg) | 0.05 | 0.06 | 0.05 |
| Retentate flowrate (g s−1) | 28 | ||
| Permeate flowrate (g s−1) | 10 | ||
| Time (min) | 9400 | ||
| Effluent | Effective Kinetic Constant | Pristine Membrane | Cleaned Membrane | Model Adjust |
|---|---|---|---|---|
| Saline effluent as received | Kpart | 26.33 | 185.55 |  |
| Kppt | 1.81 × 10−6 | 4.87 × 10−7 | ||
| Kcis | 3.37 × 10−2 | 6.38 × 10−2 | ||
| Filtered saline effluent | Kpart | 1.29 | 22.21 |  |
| Kppt | 1.72 × 10−5 | 2.40 × 10−7 | ||
| Kcis | 2.24 × 10−8 | 4.85 × 10−9 | ||
| Filtered + pH adjusted saline effluent | Kpart | 5.53 | 2.80 |  |
| Kppt | 2.58 × 10−7 | 8.03 × 10−7 | ||
| Kcis | 2.58 × 10−2 | 1.77 × 10−9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.G.; Scherer, C.M.; Fisch, A.G.; Rodrigues, M.A.S. Membrane Distillation: Pre-Treatment Effects on Fouling Dynamics. Membranes 2021, 11, 958. https://doi.org/10.3390/membranes11120958
Santos PG, Scherer CM, Fisch AG, Rodrigues MAS. Membrane Distillation: Pre-Treatment Effects on Fouling Dynamics. Membranes. 2021; 11(12):958. https://doi.org/10.3390/membranes11120958
Chicago/Turabian StyleSantos, Paula G., Cíntia M. Scherer, Adriano G. Fisch, and Marco Antônio S. Rodrigues. 2021. "Membrane Distillation: Pre-Treatment Effects on Fouling Dynamics" Membranes 11, no. 12: 958. https://doi.org/10.3390/membranes11120958
APA StyleSantos, P. G., Scherer, C. M., Fisch, A. G., & Rodrigues, M. A. S. (2021). Membrane Distillation: Pre-Treatment Effects on Fouling Dynamics. Membranes, 11(12), 958. https://doi.org/10.3390/membranes11120958