Mixed Matrix Membranes Based on Torlon® and ZIF-8 for High-Temperature, Size-Selective Gas Separations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer, Filler
2.2. Membranes Preparation
2.3. Morphological Characterization
2.4. Thermal Properties
2.5. Gas Permeation Experiments
3. Results
3.1. Membranes
3.2. SEM Analysis
3.3. Thermal Behaviour
3.4. Permeability and Selectivity
3.4.1. Data on Pure Torlon® and Comparison with the Literature
3.4.2. Permeability and Ideal Selectivity
| A (Barrer/Å) | B (Barrer) | R2 | |
|---|---|---|---|
| Torlon/6 ZIF-8, 35 °C | 6.23 | 21.6 | 0.991 |
| Torlon/6 ZIF-8, 65 °C | 11.0 | 38.2 | 0.966 |
| Torlon, 35 °C [49] | 6.85 | 23.7 | 0.960 |
3.4.3. Effect of Filler Loading on Diffusivity and Diffusivity–Selectivity
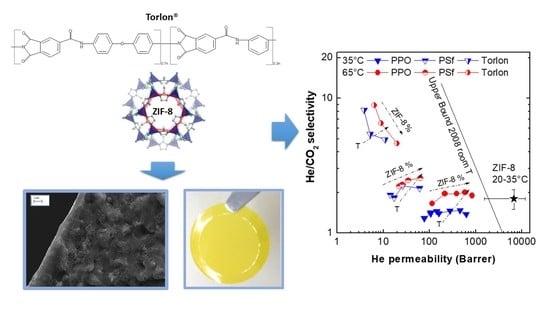
3.5. Comparison with Other Membranes Performances and with Robeson’s Upper Bound
3.6. Comparison with Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef]
- Koros, W.J.; Mahajan, R. Pushing the Limits on Possibilities for Large Scale Gas Separation: Which Strategies? J. Memb. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Henis, J.M.S.; Tripodi, M.K. A Novel Approach to Gas Separations Using Composite Hollow Fiber Membranes. Sep. Sci. Technol. 1980, 15, 1059–1068. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane Gas Separation Applications in Natural Gas Processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Rose, I.; Bezzu, C.G.; Carta, M.; Comesanã-Gándara, B.; Lasseuguette, E.; Ferrari, M.C.; Bernardo, P.; Clarizia, G.; Fuoco, A.; Jansen, J.C.; et al. Polymer Ultrapermeability from the Inefficient Packing of 2D Chains. Nat. Mater. 2017, 16, 932–937. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An Efficient Polymer Molecular Sieve for Membrane Gas Separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef]
- Budd, P.M.; Elabas, E.S.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E.; Wang, D. Solution-Processed, Organophilic Membrane Derived from a Polymer of Intrinsic Microporosity. Adv. Mater. 2004, 16, 456–459. [Google Scholar] [CrossRef]
- Lai, H.W.H.; Benedetti, F.M.; Jin, Z.; Teo, Y.C.; Wu, A.X.; De Angelis, M.G.; Smith, Z.P.; Xia, Y. Tuning the Molecular Weights, Chain Packing, and Gas-Transport Properties of CANAL Ladder Polymers by Short Alkyl Substitutions. Macromolecules 2019, 52, 6294–6302. [Google Scholar] [CrossRef]
- He, Y.; Benedetti, F.M.; Lin, S.; Liu, C.; Zhao, Y.; Ye, H.; Van Voorhis, T.; De Angelis, M.G.; Swager, T.M.; Smith, Z.P. Polymers with Side Chain Porosity for Ultrapermeable and Plasticization Resistant Materials for Gas Separations. Adv. Mater. 2019, 31, 1807871. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chaudhari, H.D.; Banerjee, R.; Kharul, U.K. Chemically Stable Covalent Organic Framework (COF)-Polybenzimidazole Hybrid Membranes: Enhanced Gas Separation through Pore Modulation. Chem. A Eur. J. 2016, 22, 4695–4699. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chemie Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Challenges and Opportunities for Mixed-Matrix Membranes for Gas Separation. J. Mater. Chem. A 2013, 1, 4610–4630. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal Organic Framework Based Mixed Matrix Membranes: An Increasingly Important Field of Research with a Large Application Potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed Matrix Membranes (MMMs) Comprising Organic Polymers with Dispersed Inorganic Fillers for Gas Separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of Graphene and Graphene Oxide Nanoplatelets on the Gas Permselectivity and Aging Behavior of Poly(Trimethylsilyl Propyne) (PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Althumayri, K.; Harrison, W.J.; Shin, Y.; Gardiner, J.M.; Casiraghi, C.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. The Influence of Few-Layer Graphene on the Gas Permeability of the High-Free-Volume Polymer PIM-1. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merkel, T.C.; He, Z.; Pinnau, I.; Freeman, B.D.; Meakin, P.; Hill, A.J. Sorption and Transport in Poly(2,2-Bis(Trifluoromethyl)-4,5-Difluoro-1,3-Dioxole- c o -Tetrafluoroethylene) Containing Nanoscale Fumed Silica. Macromolecules 2003, 36, 8406–8414. [Google Scholar] [CrossRef]
- Chi, W.S.; Sundell, B.J.; Zhang, K.; Harrigan, D.J.; Hayden, S.C.; Smith, Z.P. Mixed-Matrix Membranes Formed from Multi-Dimensional Metal–Organic Frameworks for Enhanced Gas Transport and Plasticization Resistance. Chem. Sus. Chem. 2019, 12, cssc.201900623. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The Chemistry of Metal–Organic Frameworks for CO2 Capture, Regeneration and Conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Li, J.; Sculley, J.; Zhou, H. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Yehia, H.; Pisklak, T.J.; Balkus, K.J.; Musselman, I.H. Methane Facilitated Transport Using Copper(II) Biphenyl Dicarboxylate-Triethylenediamine/Poly (3-Acetoxyethylthiophene) Mixed Matrix Membranes. In Proceedings of the Polymer Preprints–America; American Chemical Society: Washington, DC, USA, 2004; pp. 35–36. [Google Scholar]
- Mahajan, R.; Koros, W.J. Factors Controlling Successful Formation of Mixed-Matrix Gas Separation Materials. Ind. Eng. Chem. Res. 2000, 39, 2692–2696. [Google Scholar] [CrossRef]
- Mahajan, R.; Koros, W.J. Mixed Matrix Membrane Materials with Glassy Polymers. Part 1. Polym. Eng. Sci. 2002, 42, 1420–1431. [Google Scholar] [CrossRef]
- Caro, J. Are MOF Membranes Better in Gas Separation than Those Made of Zeolites? Curr. Opin. Chem. Eng. 2011, 1, 77–83. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed Matrix Membranes for Improved Gas Separation Performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Ouyang, J.; Liu, T.; Li, Z.; Li, Z.; Liu, Y.; Wang, S.; Wu, H.; Jiang, Z.; Cao, X. Enhanced Interfacial Interaction and CO2 Separation Performance of Mixed Matrix Membrane by Incorporating Polyethylenimine-Decorated Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2015, 7, 1065–1077. [Google Scholar] [CrossRef]
- Qian, Q.; Wu, A.X.; Chi, W.S.; Asinger, P.A.; Lin, S.; Hypsher, A.; Smith, Z.P. Mixed-Matrix Membranes Formed from Imide-Functionalized UiO-66-NH 2 for Improved Interfacial Compatibility. ACS Appl. Mater. Interfaces 2019, 11, 31257–31269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Bux, H.; Yang, W.; Caro, J. Zeolitic Imidazolate Framework ZIF-7 Based Molecular Sieve Membrane for Hydrogen Separation. J. Memb. Sci. 2010, 354, 48–54. [Google Scholar] [CrossRef]
- Aceituno Melgar, V.M.; Kim, J.; Othman, M.R. Zeolitic Imidazolate Framework Membranes for Gas Separation: A Review of Synthesis Methods and Gas Separation Performance. J. Ind. Eng. Chem. 2015, 28, 1–15. [Google Scholar] [CrossRef]
- Parent, L.R.; Pham, C.H.; Patterson, J.P.; Denny, M.S.; Cohen, S.M.; Gianneschi, N.C.; Paesani, F. Pore Breathing of Metal-Organic Frameworks by Environmental Transmission Electron Microscopy. J. Am. Chem. Soc. 2017, 139, 13973–13976. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, J.H.; Jung, G.Y.; Kim, Y.K.; Kim, T.K.; Jeoung, S.; Kwak, S.K.; Moon, D.; Moon, H.R. Exploration of Gate-Opening and Breathing Phenomena in a Tailored Flexible Metal–Organic Framework. Inorg. Chem. 2016, 55, 1920–1925. [Google Scholar] [CrossRef]
- Shieh, J.-J.; Chung, T.S. Gas Permeability, Diffusivity, and Solubility of Poly(4-Vinylpyridine) Film. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2851–2861. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic Imidazolate Framework Membrane with Molecular Sieving Properties by Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Varela-Guerrero, V.; Barnett, G.V.; Jeong, H.K. Synthesis of Zeolitic Imidazolate Framework Films and Membranes with Controlled Microstructures. Langmuir 2010, 26, 14636–14641. [Google Scholar] [CrossRef]
- Bux, H.; Feldhoff, A.; Cravillon, J.; Wiebcke, M.; Li, Y.-S.; Caro, J. Oriented Zeolitic Imidazolate Framework-8 Membrane with Sharp H2/C3H8 Molecular Sieve Separation. Chem. Mater. 2011, 23, 2262–2269. [Google Scholar] [CrossRef]
- Aceituno Melgar, V.M.; Ahn, H.; Kim, J.; Othman, M.R. Highly Selective Micro-Porous ZIF-8 Membranes Prepared by Rapid Electrospray Deposition. J. Ind. Eng. Chem. 2015, 21, 575–579. [Google Scholar] [CrossRef]
- Hara, N.; Yoshimune, M.; Negishi, H.; Haraya, K.; Hara, S.; Yamaguchi, T. Diffusive Separation of Propylene/Propane with ZIF-8 Membranes. J. Memb. Sci. 2014, 450, 215–223. [Google Scholar] [CrossRef]
- Chen, C.; Ozcan, A.; Yazaydin, A.O.; Ladewig, B.; Through, P.; Chemrxiv, S.Z.-M.; Chen, C.; Ozcan, A.; Yazaydin, A.O. Gas Permeation Through Single-Crystal ZIF-8 Membranes Gas Permeation through Single-Crystal ZIF-8 Membranes. J. Membr. Sci. 2019, 575, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Lively, R.P.; Zhang, K.; Johnson, J.R.; Karvan, O.; Koros, W.J. Unexpected Molecular Sieving Properties of Zeolitic Imidazolate Framework-8. J. Phys. Chem. Lett. 2012, 3, 8–12. [Google Scholar] [CrossRef]
- Shekhah, O.; Swaidan, R.; Belmabkhout, Y.; du Plessis, M.; Jacobs, T.; Barbour, L.J.; Pinnau, I.; Eddaoudi, M. The liquid phase epitaxy approach for the successful construction of ultra-thin and defect-free ZIF-8 membranes: Pure and mixed gas transport study. Chem. Commun. 2014, 50, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Kosuri, M.R.; Koros, W.J. Defect-Free Asymmetric Hollow Fiber Membranes from Torlon®, a Polyamide-Imide Polymer, for High-Pressure CO2 Separations. J. Memb. Sci. 2008, 320, 65–72. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Chung, T.S. Carbon Membranes from Blends of PBI and Polyimides for N2/CH4 and CO2/CH4 Separation and Hydrogen Purification. J. Memb. Sci. 2009, 328, 174–185. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Chung, T.S.; Tong, Y.W. Molecular Interaction, Gas Transport Properties and Plasticization Behavior of CPIM-1/Torlon Blend Membranes. J. Memb. Sci. 2014, 462, 119–130. [Google Scholar] [CrossRef]
- Vaughn, J.; Koros, W.J. Effect of the Amide Bond Diamine Structure on the CO 2, H 2S, and CH 4 Transport Properties of a Series of Novel 6FDA-Based Polyamide-Imides for Natural Gas Purification. Macromolecules 2012, 45, 7036–7049. [Google Scholar] [CrossRef]
- Merkel, T.C.; Zhou, M.; Baker, R.W. Carbon Dioxide Capture with Membranes at an IGCC Power Plant. J. Memb. Sci. 2012, 389, 441–450. [Google Scholar] [CrossRef]
- Marano, J.J.; Ciferino, J.P. Integration of Gas Separation Membranes with IGCC Identifying the Right Membrane for the Right Job. Energy Procedia 2009, 1, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Dibrov, G.; Ivanov, M.; Semyashkin, M.; Sudin, V.; Kagramanov, G. High-Pressure Aging of Asymmetric Torlon® Hollow Fibers for Helium Separation from Natural Gas. Fibers 2018, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.Y.; Johnson, J.R.; Ma, Y.; Mathias, R.; Bhandari, D.A.; Lively, R.P. Torlon® Hollow Fiber Membranes for Organic Solvent Reverse Osmosis Separation of Complex Aromatic Hydrocarbon Mixtures. AIChE J. 2019, 65, 1–13. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.S.; Wang, K.Y.; Guiver, M.D. Exploring Torlon/P84 Co-Polyamide-Imide Blended Hollow Fibers and Their Chemical Cross-Linking Modifications for Pervaporation Dehydration of Isopropanol. Sep. Purif. Technol. 2008, 61, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Goh, S.H.; Chung, T.S. Miscibility Study of Torlon® Polyamide-Imide with Matrimid® 5218 Polyimide and Polybenzimidazole. Polymer 2007, 48, 2901–2909. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular Sieving Realized with ZIF-8/Matrimid® Mixed-Matrix Membranes. J. Memb. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Song, Q.; Nataraj, S.K.; Roussenova, M.V.; Tan, J.C.; Hughes, D.J.; Li, W.; Bourgoin, P.; Alam, M.A.; Cheetham, A.K.; Al-Muhtaseb, S.A.; et al. Zeolitic Imidazolate Framework (ZIF-8) Based Polymer Nanocomposite Membranes for Gas Separation. Energy Environ. Sci. 2012, 5, 8359. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, Y.; Chung, T.-S. Poly-/Metal-Benzimidazole Nano-Composite Membranes for Hydrogen Purification. Energy Environ. Sci. 2011, 4, 4171. [Google Scholar] [CrossRef]
- Yang, T.; Chung, T.-S. High Performance ZIF-8/PBI Nano-Composite Membranes for High Temperature Hydrogen Separation Consisting of Carbon Monoxide and Water Vapor. Int. J. Hydrogen Energy 2013, 38, 229–239. [Google Scholar] [CrossRef]
- Yang, T.; Shi, G.M.; Chung, T.-S. Symmetric and Asymmetric Zeolitic Imidazolate Frameworks (ZIFs)/Polybenzimidazole (PBI) Nanocomposite Membranes for Hydrogen Purification at High Temperatures. Adv. Energy Mater. 2012, 2, 1358–1367. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High Performance ZIF-8/6FDA-DAM Mixed Matrix Membrane for Propylene/Propane Separations. J. Memb. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- Díaz, K.; López-González, M.; del Castillo, L.F.; Riande, E. Effect of Zeolitic Imidazolate Frameworks on the Gas Transport Performance of ZIF8-Poly(1,4-Phenylene Ether-Ether-Sulfone) Hybrid Membranes. J. Memb. Sci. 2011, 383, 206–213. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Carolus Jansen, J.; et al. Gas Permeation Parameters of Mixed Matrix Membranes Based on the Polymer of Intrinsic Microporosity PIM-1 and the Zeolitic Imidazolate Framework ZIF-8. J. Memb. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved Operational Stability of Pebax-Based Gas Separation Membranes with ZIF-8: A Comparative Study of Flat Sheet and Composite Hollow Fibre Membranes. J. Memb. Sci. 2017, 524, 266–279. [Google Scholar] [CrossRef]
- Ma, X.; Swaidan, R.J.; Wang, Y.; Hsiung, C.; Han, Y.; Pinnau, I. Highly Compatible Hydroxyl-Functionalized Microporous Polyimide-ZIF-8 Mixed Matrix Membranes for Energy Efficient Propylene/Propane Separation. ACS Appl. Nano Mater. 2018, 1, 3541–3547. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of Metal-Organic Frameworks by Water Adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Freitas, A.P.; Boutin, A.; Fuchs, A.H.; Coudert, F.X. What Makes Zeolitic Imidazolate Frameworks Hydrophobic or Hydrophilic? The Impact of Geometry and Functionalization on Water Adsorption. Phys. Chem. Chem. Phys. 2014, 16, 9940–9949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, F.M.; De Angelis, M.G.; Esposti, M.D.; Fabbri, P.; Masili, A.; Orsini, A.; Pettinau, A. Enhancing the Separation Performance of Glassy PPO with the Addition of a Molecular Sieve (ZIF-8): Gas Transport at Various Temperatures. Membranes 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Papchenko, K.; Risaliti, G.; Ferroni, M.; Christian, M.; De Angelis, M.G. An Analysis of the Effect of Zif-8 Addition on the Separation Properties of Polysulfone at Various Temperatures. Membranes 2021, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Forman, E.M.; Baniani, A.; Fan, L.; Ziegler, K.J.; Zhou, E.; Zhang, F.; Lively, R.P.; Vasenkov, S. Relationship between Ethane and Ethylene Diffusion inside ZIF-11 Crystals Confined in Polymers to Form Mixed-Matrix Membranes. J. Memb. Sci. 2020, 593, 117440. [Google Scholar] [CrossRef]
- Forman, E.M.; Baniani, A.; Fan, L.; Ziegler, K.J.; Zhou, E.; Zhang, F.; Lively, R.P.; Vasenkov, S. Ethylene Diffusion in Crystals of Zeolitic Imidazole Framework-11 Embedded in Polymers to Form Mixed-Matrix Membranes. Microporous Mesoporous Mater. 2019, 274, 163–170. [Google Scholar] [CrossRef]
- Das, M.; Perry, J.D.; Koros, W.J. Gas-Transport-Property Performance of Hybrid Carbon Molecular Sieve−Polymer Materials. Ind. Eng. Chem. Res. 2010, 49, 9310–9321. [Google Scholar] [CrossRef]
- Horn, N.R.; Paul, D.R. Carbon Dioxide Plasticization and Conditioning Effects in Thick vs. Thin Glassy Polymer Films. Polymer 2011, 52, 1619–1627. [Google Scholar] [CrossRef]
- Bos, A.; Pünt, I.G.M.; Wessling, M.; Strathmann, H. CO2-Induced Plasticization Phenomena in Glassy Polymers. J. Memb. Sci. 1999, 155, 67–78. [Google Scholar] [CrossRef]
- Handa, Y.P.; Lampron, S.; O’neill, M.L. On the Plasticization of Poly(2,6-Dimethyl Phenylene Oxide) by CO2. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 2549–2553. [Google Scholar] [CrossRef] [Green Version]
- Minelli, M.; Deangelis, M.; Doghieri, F.; Marini, M.; Toselli, M.; Pilati, F. Oxygen Permeability of Novel Organic–Inorganic Coatings: I. Effects of Organic–Inorganic Ratio and Molecular Weight of the Organic Component. Eur. Polym. J. 2008, 44, 2581–2588. [Google Scholar] [CrossRef]
- Bevington, P.R.; Robinson, D.K. Error Data Reduction and Error Analysis for the Physical Sciences, 3rd ed.; McGraw-Hill: New York, NY, USA, 1992; Volume 7, ISBN 0072472278. [Google Scholar]
- Daynes, H.A. The Process of Diffusion through a Rubber Membrane. Proc. R. Soc. A Math. Phys. Eng. Sci. 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; ISBN 978-0198534112. [Google Scholar]
- Science, M. Permeation through a Heterogeneous Membrane: The Effect of the Dispersed Phase. J. Membr. Sci. 1997, 128, 141–149. [Google Scholar]







| Polymer | Density at 25 °C (g/cm3) | Tg (°C) | FFV (%) |
|---|---|---|---|
| Torlon® 4000 TF | 1.252 [50] | 274–276 (DSC, [57]) 281–292 (DMA, [57]) | 8 [50] |
| Filler | Composition [68] | Net [60] | da, Å [34] | dp, Å [34] | Surface Area (BET), m2/g [68] | Theoretical Density, g/cm3 | Thermal Stability, °C [68] | Hydrophilicity [68,69] |
|---|---|---|---|---|---|---|---|---|
| ZIF-8 | Zn(MeIM)2 | sod | 3.4 | 11.6 | 1630 | 0.95 [59] 0.93 [65] | 550 | Hydrophobic |
| Tg (°C) | ||
|---|---|---|
| Torlon | 267 | |
| Torlon/6 ZIF-8 | 297 | |
| Torlon/15 ZIF-8 | 296 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pascale, M.; Benedetti, F.M.; Lasseuguette, E.; Ferrari, M.-C.; Papchenko, K.; Degli Esposti, M.; Fabbri, P.; De Angelis, M.G. Mixed Matrix Membranes Based on Torlon® and ZIF-8 for High-Temperature, Size-Selective Gas Separations. Membranes 2021, 11, 982. https://doi.org/10.3390/membranes11120982
De Pascale M, Benedetti FM, Lasseuguette E, Ferrari M-C, Papchenko K, Degli Esposti M, Fabbri P, De Angelis MG. Mixed Matrix Membranes Based on Torlon® and ZIF-8 for High-Temperature, Size-Selective Gas Separations. Membranes. 2021; 11(12):982. https://doi.org/10.3390/membranes11120982
Chicago/Turabian StyleDe Pascale, Matilde, Francesco Maria Benedetti, Elsa Lasseuguette, Maria-Chiara Ferrari, Kseniya Papchenko, Micaela Degli Esposti, Paola Fabbri, and Maria Grazia De Angelis. 2021. "Mixed Matrix Membranes Based on Torlon® and ZIF-8 for High-Temperature, Size-Selective Gas Separations" Membranes 11, no. 12: 982. https://doi.org/10.3390/membranes11120982
APA StyleDe Pascale, M., Benedetti, F. M., Lasseuguette, E., Ferrari, M. -C., Papchenko, K., Degli Esposti, M., Fabbri, P., & De Angelis, M. G. (2021). Mixed Matrix Membranes Based on Torlon® and ZIF-8 for High-Temperature, Size-Selective Gas Separations. Membranes, 11(12), 982. https://doi.org/10.3390/membranes11120982