Higher Acid Recovery Efficiency of Novel Functionalized Inorganic/Organic Composite Anion Exchange Membranes from Acidic Wastewater
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Quaternized poly (2,6-dimethyl-1,4-phenylene Oxide) Membrane
2.3. Preparation of the Functionalized Inorganic/Organic Composite AEMs
2.4. Characterizations
2.4.1. Instrumentations
2.4.2. Water Uptake and Linear Swelling Ratio
2.4.3. Ion Exchange Capacity
2.4.4. Acidic Stability Test
2.4.5. Diffusion Dialysis Test
3. Results and Discussions
3.1. FTIR
3.2. Morphology Test
3.3. Ion Exchange Capacity
3.4. Water Uptake and Linear Swelling Ratio
3.5. Mechanical and Thermal Stability
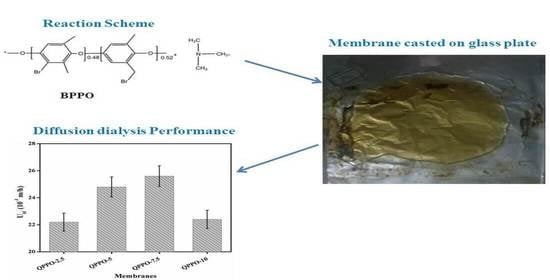
3.6. Acid Recovery Performance
3.7. Acid Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Codes | Full names |
| AEM | Anion exchange membrane |
| BPPO | Brominated poly(2,6-dimethyl-1,4-phenylene oxide) |
| DD | Diffusion dialysis |
| TMSP-TMA+Cl− | N-(trimethoxysilylpropyl)-N,N,N-trimethylammonium chloride |
| IEC | Ion exchange capacity |
| IEM | Ion exchange membrane |
References
- Agrawal, A.; Sahu, K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.; Wiśniewska, G. Water and acid recovery from the rinse after metal etching operations. Hydrometallurgy 1999, 53, 105–119. [Google Scholar] [CrossRef]
- Naik, N.S.; Padaki, M.; Déon, S.; Murthy, D.H.K. Novel poly (ionic liquid)-based anion exchange membranes for efficient and rapid acid recovery from industrial waste. Chem. Eng. J. 2020, 401, 126148. [Google Scholar] [CrossRef]
- Xiao, H.-F.; Chen, Q.; Cheng, H.; Li, X.-M.; Qin, W.-M.; Chen, B.-S.; Xiao, D.; Zhang, W.-M. Selective removal of halides from spent zinc sulfate electrolyte by diffusion dialysis. J. Membr. Sci. 2017, 537, 111–118. [Google Scholar] [CrossRef]
- Stachera, D.M.; Childs, R.F.; Mika, A.M.; Dickson, J.M. Acid recovery using diffusion dialysis with poly (4-vinylpyridine)-filled microporous membranes. J. Membr. Sci. 1998, 148, 119–127. [Google Scholar] [CrossRef]
- Sridhar, P.; Subramaniam, G. Recovery of acid from cation exchange resin regeneration waste by diffusion dialysis. J. Membr. Sci. 1989, 45, 273–280. [Google Scholar] [CrossRef]
- Xu, J.; Lu, S.; Fu, D. Recovery of hydrochloric acid from the waste acid solution by diffusion dialysis. J. Hazard. Mater. 2009, 165, 832–837. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, X.; Hickner, M.A. Exploring backbone-cation alkyl spacers for multi-cation side chain anion exchange membranes. J. Power Sources 2018, 375, 433–441. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Z.; Pan, J.; Tong, B.; Xu, T. Facile and cost effective pva based hybrid membrane fabrication for acid recovery. Sep. Purif. Technol. 2014, 136, 250–257. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Industrial recovery of mixed acid (HF+HNO3) from the titanium spent leaching solutions by diffusion dialysis with a new series of anion exchange membranes. J. Membr. Sci. 2003, 220, 89–95. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Continuous dialysis of sulphuric acid and sodium sulphate mixture. J. Membr. Sci. 2016, 497, 36–46. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Li, Z.; Liao, J.; Huang, Y.; Lei, Y.; Li, N. Mixed-charge poly(2,6-dimethyl-phenylene oxide)anion exchange membrane for diffusion dialysis in acid recovery. J. Membr. Sci. 2018, 549, 543–549. [Google Scholar] [CrossRef]
- Vivek Chavan, C.A.; Adya, V.C. Ashok Kumar Pandey. Hybrid organic-inorganic anion-exchange pore-filled membranes for the recovery of nitric acid from highly acidic aqueous waste streams. Water Res. 2018, 133, 87–98. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Continuous dialysis of mixture of inorganic acids. Sep. Purif. Technol. 2017, 172, 277–284. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Reddy, N.N.; Nimiwal, R.; Singh, P.S.; Adimurthy, S.; Nagarale, R.K. Polyaniline@porous polypropylene for efficient separation of acid by diffusion dialysis. Sep. Purif. Technol. 2020, 233, 115989. [Google Scholar] [CrossRef]
- He, Y.; Pan, J.; Wu, L.; Ge, L.; Xu, T. Facile preparation of 1,8-diazabicyclo[5.4.0]undec-7-ene based high performance anion exchange membranes for diffusion dialysis applications. J. Membr. Sci. 2015, 491, 45–52. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Separation of HCl+FeCl2 mixture by anion-exchange membrane. Sep. Purif. Technol. 2009, 66, 45–50. [Google Scholar] [CrossRef]
- Ge, Q.; Ning, Y.; Wu, L.; Ge, L.; Liu, X.; Yang, Z.; Xu, T. Enhancing acid recovery efficiency by implementing oligomer ionic bridge in the membrane matrix. J. Membr. Sci. 2016, 518, 263–272. [Google Scholar] [CrossRef]
- Pan, J.; He, Y.; Wu, L.; Jiang, C.; Wu, B.; Mondal, A.N.; Cheng, C.; Xu, T. Anion exchange membranes from hot-pressed electrospun QPPO–SiO2 hybrid nanofibers for acid recovery. J. Membr. Sci. 2015, 480, 115–121. [Google Scholar] [CrossRef]
- Yadav, V.; Raj, S.K.; Rathod, N.H.; Kulshrestha, V. Polysulfone/graphene quantum dots composite anion exchange membrane for acid recovery by diffusion dialysis. J. Membr. Sci. 2020, 611, 118331. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Ran, J.; Wu, L.; Ru, Y.; Hu, M.; Din, L.; Xu, T. Anion exchange membranes (AEMs) based on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) and its derivatives. Polym. Chem. 2015, 6, 5809–5926. [Google Scholar] [CrossRef]
- Li, N.; Guiver, M.D. Ion transport by nanochannels in ion-containing aromatic copolymers. Macromolecules 2014, 47, 2175–2198. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Khraisheh, M.; Almomani, F. Fabrication and characterization of pyridinium functionalized anion exchange membranes for acid recovery. Sci. Total Environ. 2019, 686, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Q.; Wang, C.; Lee, Y.M.; Guiver, M.D. Phenyltrimethylammonium functionalized polysulfone anion exchange membranes. Macromolecules 2012, 45, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Varcoe, J.R.; Slade, R.C.T.; Lam How Yee, E.; Poynton, S.D.; Driscoll, D.J.; Apperley, D.C. Poly(ethylene-co-tetrafluoroethylene)-derived radiation-grafted anion-exchange membrane with properties specifically tailored for application in metal-cation-free alkaline polymer electrolyte fuel cells. Chem. Mater. 2007, 19, 2686–2693. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Erigene, B.; Mondal, A.N.; Hossain, M.M.; Khan, M.I.; Afsar, N.U.; Liang, G.; Wu, L.; Xu, T. Imidazolium functionalized anion exchange membrane blended with PVA for acid recovery via diffusion dialysis process. J. Membr. Sci. 2016, 497, 209–215. [Google Scholar] [CrossRef]
- Song, F.; Fu, Y.; Gao, Y.; Li, J.; Qiao, J.; Zhou, X.-D.; Liu, Y. Novel alkaline anion-exchange membranes based on chitosan/ethenylmethylimidazoliumchloride polymer with ethenylpyrrolidone composites for low temperature polymer electrolyte fuel cells. Electrochim. Acta 2015, 177, 137–144. [Google Scholar] [CrossRef]
- Chen, N.; Zhu, H.; Chu, Y.; Li, R.; Liu, Y.; Wang, F. Cobaltocenium-containing polybenzimidazole polymers for alkaline anion exchange membrane applications. Polym. Chem. 2017, 8, 1381–1392. [Google Scholar] [CrossRef]
- Khan, M.I.; Luque, R.; Prinsen, P.; Rehman, A.; Anjum, S.; Nawaz, M.; Shaheen, A.; Zafar, S.; Mustaqeem, M. BPPO-based anion exchange membranes for acid recovery via diffusion dialysis. Materials 2017, 10, 266. [Google Scholar] [CrossRef]
- Khan, M.I.; Luque, R.; Akhtar, S.; Shaheen, A.; Mehmood, A.; Idress, S.; Buzdar, S.; Rehman, A. Design of anion exchange membranes and electrodialysis studies for water desalination. Materials 2016, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Mondal, A.N.; Tong, B.; Jiang, C.; Emmanuel, K.; Yang, Z.; Wu, L.; Xu, T. Development of BPPO-based anion exchange membranes for electrodialysis desalination applications. Desalination 2016, 391, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Shamsaei, E.; Low, Z.-X.; Lin, X.; Mayahi, A.; Liu, H.; Zhang, X.; Zhe Liu, J.; Wang, H. Rapid synthesis of ultrathin, defect-free ZIF-8 membranes via chemical vapour modification of a polymeric support. Chem. Commun. 2015, 51, 11474–11477. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Su, J.; Lichtfouse, E.; Guo, L. Higher efficiency of triethanolamine-grafted anion exchange membranes for acidic wastewater treatment. Desal. Water Treat. 2020, 197, 41–51. [Google Scholar] [CrossRef]
- Khan, M.I.; Su, J.; Guo, L. Development of triethanolamine functionalized-anion exchange membrane for adsorptive removal of methyl orange from aqueous solution. Desal. Water Treat. 2021, 209, 342–352. [Google Scholar] [CrossRef]
- Khan, M.I.; Zheng, C.; Mondal, A.N.; Hossain, M.M.; Wu, B.; Emmanuel, K.; Wu, L.; Xu, T. Preparation of anion exchange membranes from BPPO and dimethylethanolamine for electrodialysis. Desalination 2017, 402, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Fernandez-Garcia, J.; Zhu, Q.-L. Fabrication of doubly charged anion-exchange membranes for enhancing hydroxide conductivity. Sep. Sci. Technol. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Khan, M.I. Comparison of different quaternary ammonium groups on desalination performance of bppo-based anion exchange membranes. Desal. Water Treat. 2018, 108, 49–57. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Khan, M.I.; Su, J.; Guo, L. Preparation and characterization of high-performance anion exchange membranes for acid recovery. Desal. Water Treat. 2021, 209, 144–154. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Emmanuel, K.; Hossain, M.M.; Afsar, N.U.; Wu, L.; Xu, T. Preparation of pyrrolidinium-based anion-exchange membranes for acid recovery via diffusion dialysis. Sep. Sci. Technol. 2016, 51, 1881–1890. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion exchange hybrid membranes from PVA and multi-alkoxy silicon copolymer tailored for diffusion dialysis process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Li, Y.; Xu, T.; Gong, M. Fundamental studies of a new series of anion exchange membranes: Membranes prepared from bromomethylated poly(2,6-dimethyl-1,4-phenylene oxide) (BPPO) and pyridine. J. Membr. Sci. 2006, 279, 200–208. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Yao, Z.; Pan, J.; Hossain, M.M.; Khan, M.I.; Yang, Z.; Wu, L.; Xu, T. Novel quaternized aromatic amine based hybrid PVA membranes for acid recovery. J. Membr. Sci. 2015, 490, 29–37. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M. Synthesis and characterization of stable anion exchange membranes for desalination applications. Desal. Water Treat. 2018, 113, 36–44. [Google Scholar] [CrossRef]
- Hossain, M.M.; Hou, J.; Wu, L.; Ge, Q.; Liang, X.; Mondal, A.N.; Xu, T. Anion exchange membranes with clusters of alkyl ammonium group for mitigating water swelling but not ionic conductivity. J. Membr. Sci. 2018, 550, 101–109. [Google Scholar] [CrossRef]
- Hossain, M.M.; Wu, L.; Liang, X.; Yang, Z.; Hou, J.; Xu, T. Anion exchange membrane crosslinked in the easiest way stands out for fuel cells. J. Power Sources 2018, 390, 234–241. [Google Scholar] [CrossRef]
- Mondal, A.N.; He, Y.; Wu, L.; Khan, M.I.; Emmanuel, K.; Hossain, M.M.; Ge, L.; Xu, T. Click mediated high-performance anion exchange membranes with improved water uptake. J. Mater. Chem. A 2017, 5, 1022–1027. [Google Scholar] [CrossRef]
- Khan, M.I.; Wu, L.; Hossain, M.M.; Pan, J.; Ran, J.; Mondal, A.N.; Xu, T. Preparation of diffusion dialysis membrane for acid recovery via a phase-inversion method. Membr. Water Treat. 2015, 6, 365–378. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Wu, C.; Xu, T.; Fu, Y. Bionic multisilicon copolymers used as novel cross-linking agents for preparing anion exchange hybrid membranes. J. Phys. Chem. B 2011, 115, 6474–6483. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Wu, Y.; Xu, T. Diffusion dialysis of hydrochloride acid at different temperatures using ppo–sio 2 hybrid anion exchange membranes. J. Membr. Sci. 2010, 347, 240–249. [Google Scholar] [CrossRef]









| Membranes | TS (MPa) | Eb (%) | λ | Thickness (μm) |
|---|---|---|---|---|
| QPPO | 19 ± 0.95 | 10 ± 0.50 | 10.40 ± 0.52 | 62 ± 3.10 |
| QPPO-2.5 | 48 ± 2.40 | 27 ± 1.35 | 17.72 ± 0.87 | 67 ± 3.35 |
| QPPO-5 | 39 ± 1.95 | 41 ± 2.10 | 18.11 ± 0.91 | 61 ± 3.10 |
| QPPO-7.5 | 32 ± 1.60 | 56 ± 2.80 | 19.80 ± 1.00 | 73 ± 3.65 |
| QPPO-10 | 22 ± 1.10 | 14 ± 0.70 | 11.50 ± 0.58 | 59 ± 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.I.; Shanableh, A.; Elboughdiri, N.; Kriaa, K.; Ghernaout, D.; Ghareba, S.; Khraisheh, M.; Lashari, M.H. Higher Acid Recovery Efficiency of Novel Functionalized Inorganic/Organic Composite Anion Exchange Membranes from Acidic Wastewater. Membranes 2021, 11, 133. https://doi.org/10.3390/membranes11020133
Khan MI, Shanableh A, Elboughdiri N, Kriaa K, Ghernaout D, Ghareba S, Khraisheh M, Lashari MH. Higher Acid Recovery Efficiency of Novel Functionalized Inorganic/Organic Composite Anion Exchange Membranes from Acidic Wastewater. Membranes. 2021; 11(2):133. https://doi.org/10.3390/membranes11020133
Chicago/Turabian StyleKhan, Muhammad Imran, Abdallah Shanableh, Noureddine Elboughdiri, Karim Kriaa, Djamel Ghernaout, Saad Ghareba, Majeda Khraisheh, and Mushtaq Hussain Lashari. 2021. "Higher Acid Recovery Efficiency of Novel Functionalized Inorganic/Organic Composite Anion Exchange Membranes from Acidic Wastewater" Membranes 11, no. 2: 133. https://doi.org/10.3390/membranes11020133
APA StyleKhan, M. I., Shanableh, A., Elboughdiri, N., Kriaa, K., Ghernaout, D., Ghareba, S., Khraisheh, M., & Lashari, M. H. (2021). Higher Acid Recovery Efficiency of Novel Functionalized Inorganic/Organic Composite Anion Exchange Membranes from Acidic Wastewater. Membranes, 11(2), 133. https://doi.org/10.3390/membranes11020133